Photocatalyzed Cascade Reactions of Cyclopropanols and α-Trifluoromethyl-Substituted Olefins for the Synthesis of Fused gem-Difluorooxetanes
Dr. Yunxiao Zhang
Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei 430072 China
These authors contributed equally to this work.
Search for more papers by this authorYunhong Niu
Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei 430072 China
These authors contributed equally to this work.
Search for more papers by this authorYouyuan Guo
Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei 430072 China
Search for more papers by this authorJiaxin Wang
Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei 430072 China
Search for more papers by this authorYizhi Zhang
Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei 430072 China
Search for more papers by this authorDr. Shanshan Liu
Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei 430072 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiao Shen
Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei 430072 China
Search for more papers by this authorDr. Yunxiao Zhang
Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei 430072 China
These authors contributed equally to this work.
Search for more papers by this authorYunhong Niu
Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei 430072 China
These authors contributed equally to this work.
Search for more papers by this authorYouyuan Guo
Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei 430072 China
Search for more papers by this authorJiaxin Wang
Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei 430072 China
Search for more papers by this authorYizhi Zhang
Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei 430072 China
Search for more papers by this authorDr. Shanshan Liu
Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei 430072 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiao Shen
Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei 430072 China
Search for more papers by this authorGraphical Abstract
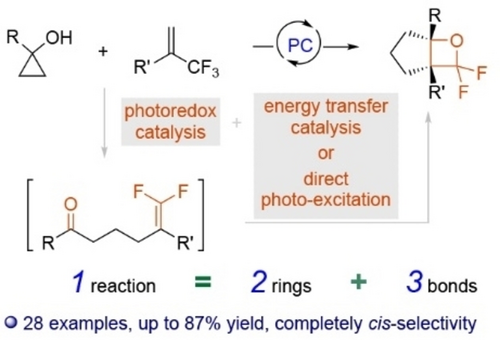
A combination of photoredox catalysis and energy transfer catalysis/direct photo-excitation enables cascade reactions of cyclopropanols and α-CF3-olefins, in which two rings and three bonds are constructed in one reaction, affording fused gem-difluorooxetanes. The reaction shows broad substrate scope, complete cis-selectivity, and downstream transformations of the products demonstrated the synthetic potential.
Abstract
Fluorinated fused rings are challenging to construct from simple starting materials. Herein, we report the first photocatalyzed cascade reactions of readily available cyclopropanols and α-trifluoromethyl-substituted olefins for the synthesis of fused gem-difluorooxetanes. Two rings and three bonds were efficiently constructed in one reaction. The reaction showed broad substrate scope and the downstream transformations of the products demonstrated the synthetic potential of the reaction. The mechanistic study supported the presence of cascade photoredox catalysis and energy transfer catalysis/direct photo-excitation processes.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202212201-sup-0001-20220909-NYH-8-081_auto.cif333.6 KB | Supporting Information |
| anie202212201-sup-0001-misc_information.pdf6.7 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see
- 1aR. D. Taylor, M. MacCoss, A. D. G. Lawson, J. Med. Chem. 2014, 57, 5845–5859;
- 1bJ. Shearer, J. L. Castro, A. D. G. Lawson, M. MacCoss, R. D. Taylor, J. Med. Chem. 2022, 65, 8699–8712.
- 2For reviews of oxetanes in medicinal chemistry, see
- 2aJ. A. Burkhard, G. Wuitschik, M. Rogers-Evans, K. Müller, E. M. Carreira, Angew. Chem. Int. Ed. 2010, 49, 9052–9067; Angew. Chem. 2010, 122, 9236–9251;
- 2bG. Wuitschik, E. M. Carreira, B. Wagner, H. Fischer, I. Parrilla, F. Schuler, M. Rogers-Evans, K. Müller, J. Med. Chem. 2010, 53, 3227–3246;
- 2cJ. A. Bull, R. A. Croft, O. A. Davis, R. Doran, K. F. Morgan, Chem. Rev. 2016, 116, 12150–12233.
- 3For reviews on the synthesis and applications of oxetanes in synthetic chemistry, see
- 3aT. Bach, Synthesis 1998, 683–703;
- 3bP. H. Dussault, C. Xu, in Comprehensive Heterocyclic Chemistry III (Eds.: A. R. Katritzky, C. A. Ramsden, E. F. V. Scriven, R. J. K. Taylor), Elsevier, Oxford, 2008, pp. 365–387;
- 3cM. Abe, J. Chin. Chem. Soc. 2008, 55, 479–486;
- 3dM. D′Auria, R. Racioppi, Molecules 2013, 18, 11384–11428;
- 3eZ. Wang, Z. Chen, J. Sun, Org. Biomol. Chem. 2014, 12, 6028–6032;
- 3fA. Mahal, Eur. J. Chem. 2015, 6, 357–366;
- 3gC. A. Malapit, A. R. Howell, J. Org. Chem. 2015, 80, 8489–8495;
- 3hM. D′Auria, Photochem. Photobiol. Sci. 2019, 18, 2297–2362.
- 4
- 4aG. Wuitschik, M. Rogers-Evans, K. Müller, H. Fischer, B. Wagner, F. Schuler, L. Polonchuk, E. M. Carreira, Angew. Chem. Int. Ed. 2006, 45, 7736–7739; Angew. Chem. 2006, 118, 7900–7903;
- 4bG. Wuitschik, Oxetanes in Drug Discovery, Ph.D. Thesis, ETH Zurich, 2008.
- 5
- 5aJ. Hu, W. Zhang, F. Wang, Chem. Commun. 2009, 7465–7478;
- 5bD. E. Yerien, S. Barata-Vallejo, A. Postigo, Chem. Eur. J. 2017, 23, 14676–14701;
- 5cJ. B. I. Sap, C. F. Meyer, N. J. W. Straathof, N. Iwumene, C. W. am Ende, A. A. Trabanco, V. Gouverneur, Chem. Soc. Rev. 2021, 50, 8214–8247;
- 5dR. Britton, V. Gouverneur, J.-H. Lin, M. Meanwell, C. Ni, G. Pupo, J.-C. Xiao, J. Hu, Nat. Rev. Methods Primers 2021, 1, 47;
- 5eJ. Feng, X. Jia, S. Zhang, K. Lu, D. Cahard, Org. Chem. Front. 2022, 9, 3598–3623.
- 6
- 6aN. Chauret, D. Guay, C. Li, S. Day, J. Silva, M. Blouin, Y. Ducharme, J. A. Yergey, D. A. Nicoll-Griffith, Bioorg. Med. Chem. Lett. 2002, 12, 2149–2152;
- 6bJ. W. Lee, K. N. Lee, M.-Y. Ngai, Angew. Chem. Int. Ed. 2019, 58, 11171–11181; Angew. Chem. 2019, 131, 11289–11299;
- 6cA. Loison, F. Toulgoat, T. Billard, G. Hanquet, A. Panossian, F. R. Leroux, Tetrahedron 2021, 99, 132458.
- 7V. A. Petrov, F. Davidson, B. E. Smart, J. Fluorine Chem. 2004, 125, 1543–1552.
- 8
- 8aH. Zhao, X. Fan, J. Yu, C. Zhu, J. Am. Chem. Soc. 2015, 137, 3490–3493;
- 8bK. Jia, F. Zhang, H. Huang, Y. Chen, J. Am. Chem. Soc. 2016, 138, 1514–1517;
- 8cR. Ren, Z. Wu, Y. Xu, C. Zhu, Angew. Chem. Int. Ed. 2016, 55, 2866–2869; Angew. Chem. 2016, 128, 2916–2919.
- 9
- 9aS. B. Lang, R. J. Wiles, C. B. Kelly, G. A. Molander, Angew. Chem. Int. Ed. 2017, 56, 15073–15077; Angew. Chem. 2017, 129, 15269–15273;
- 9bY. Lan, F. Yang, C. Wang, ACS Catal. 2018, 8, 9245–9251;
- 9cX. Lu, X.-X. Wang, T.-J. Gong, J.-J. Pi, S.-J. He, Y. Fu, Chem. Sci. 2019, 10, 809–814;
- 9dP.-J. Xia, Z.-P. Ye, Y.-Z. Hu, D. Song, H.-Y. Xiang, X.-Q. Chen, H. Yang, Org. Lett. 2019, 21, 2658–2662;
- 9eD. Anand, Z. Sun, L. Zhou, Org. Lett. 2020, 22, 2371–2375.
- 10
- 10aC. Hammaecher, C. Portella, Chem. Commun. 2008, 5833–5835;
- 10bJ. R. Vale, A. Valkonen, C. A. M. Afonso, N. R. Candeias, Org. Chem. Front. 2019, 6, 3793–3798.
- 11
- 11aJ. F. Harris, D. D. Coffman, J. Am. Chem. Soc. 1962, 84, 1553–1561;
- 11bE. R. Bissell, D. B. Fields, J. Org. Chem. 1964, 29, 249–252;
- 11cE. W. Cook, B. F. Landrum, J. Heterocycl. Chem. 1965, 2, 327–328;
- 11dM. G. Barlow, B. Coles, R. N. Haszeldine, J. Chem. Soc. Perkin Trans. 1 1980, 2258–2267;
- 11eG. Bargigia, C. Tonelli, M. Tato, J. Fluorine Chem. 1987, 36, 449–459;
- 11fP. Tarrant, R. N. Bull, J. Fluorine Chem. 1988, 40, 201–215;
- 11gM. A. Gomez Fernandez, C. Lefebvre, A. Sudau, P. Genix, J.-P. Vors, M. Abe, N. Hoffmann, Chem. Eur. J. 2021, 27, 15722–15729.
- 12For a thematic issue on photochemical catalytic processes, see Chem. Rev. 2022, 122, 1483–2980.
- 13For recent reviews on proton-coupled electron transfer, see
- 13aR. Tyburski, T. Liu, S. D. Glover, L. Hammarström, J. Am. Chem. Soc. 2021, 143, 560–576;
- 13bP. R. D. Murray, J. H. Cox, N. D. Chiappini, C. B. Roos, E. A. McLoughlin, B. G. Hejna, S. T. Nguyen, H. H. Ripberger, J. M. Ganley, E. Tsui, N. Y. Shin, B. Koronkiewicz, G. Qiu, R. R. Knowles, Chem. Rev. 2022, 122, 2017–2291;
- 13cR. G. Agarwal, S. C. Coste, B. D. Groff, A. M. Heuer, H. Noh, G. A. Parada, C. F. Wise, E. M. Nichols, J. J. Warren, J. M. Mayer, Chem. Rev. 2022, 122, 1–49;
- 13dD. G. Nocera, J. Am. Chem. Soc. 2022, 144, 1069–1081.
- 14Q.-Q. Zhou, Y.-Q. Zou, L.-Q. Lu, W.-J. Xiao, Angew. Chem. Int. Ed. 2019, 58, 1586–1604; Angew. Chem. 2019, 131, 1600–1619.
- 15C. K. Prier, D. A. Rankic, D. W. C. MacMillan, Chem. Rev. 2013, 113, 5322–5363.
- 16Y. Zhang, Y. Zhang, Y. Guo, S. Liu, X. Shen, Chem Catalysis 2022, 2, 1380–1393.
- 17K. Teegardin, J. I. Day, J. Chan, J. Weaver, Org. Process Res. Dev. 2016, 20, 1156–1163.
- 18For selected examples of photo-induced reactions of acylsilanes though carbene intermediates, see
- 18aK. Ito, H. Tamashima, N. Iwasawa, H. Kusama, J. Am. Chem. Soc. 2011, 133, 3716–3719;
- 18bP. Becker, D. L. Priebbenow, R. Pirwerdjan, C. Bolm, Angew. Chem. Int. Ed. 2014, 53, 269–271; Angew. Chem. 2014, 126, 273–275;
- 18cJ. H. Ye, L. Quach, T. Paulisch, F. Glorius, J. Am. Chem. Soc. 2019, 141, 16227–16231;
- 18dB. Huang, M. Wei, E. Vargo, Y. Qian, T. Xu, F. D. Toste, J. Am. Chem. Soc. 2021, 143, 17920–17925;
- 18eC. Stuckhardt, M. Wissing, A. Studer, Angew. Chem. Int. Ed. 2021, 60, 18605–18611; Angew. Chem. 2021, 133, 18753–18759;
- 18fY. Zhang, G. Zhou, X. Gong, Z. Guo, X. Qi, X. Shen, Angew. Chem. Int. Ed. 2022, 61, e202202175; Angew. Chem. 2022, 134, e202202175;
- 18gG. Zhou, X. Shen, Angew. Chem. Int. Ed. 2022, 61, e202115334; Angew. Chem. 2022, 134, e202115334;
- 18hY. Ueda, Y. Masuda, T. Iwai, K. Imaeda, H. Takeuchi, K. Ueno, M. Gao, Y.-Y. Hasegawa, M. Sawamura, J. Am. Chem. Soc. 2022, 144, 2218–2224;
- 18iZ. Zhu, W. Zhang, Y. Zhang, S. Liu, X. Shen, CCS Chem. 2022, https://doi.org/10.31635/ccschem.022.202202199;
- 18jA. Bunyamin, C. Hua, A. Polyzos, D. L. Priebbenow, Chem. Sci. 2022, 13, 3273–3280; for a recent review on siloxycarbenes, see
- 18kD. L. Priebbenow, Adv. Synth. Catal. 2020, 362, 1927–1946.
- 19For recent reviews on triplet energy transfer catalysis, see ref 10 and
- 19aF. Strieth-Kalthoff, M. J. James, M. Teders, L. Pitzer, F. Glorius, Chem. Soc. Rev. 2018, 47, 7190–7202;
- 19bM. R. Schreier, X. Guo, B. Pfund, Y. Okamoto, T. R. Ward, C. Kerzig, O. S. Wenger, Acc. Chem. Res. 2022, 55, 1290–1300;
- 19cT. Neveselý, M. Wienhold, J. J. Molloy, R. Gilmour, Chem. Rev. 2022, 122, 2650–2694;
- 19dP. Rana, N. Singh, P. Majumdar, S. Prakash Singh, Coord. Chem. Rev. 2022, 470, 214698;
- 19eR. Hojo, A. M. Polgar, Z. M. Hudson, ACS Sustainable Chem. Eng. 2022, 10, 9665–9678.
- 20For recent examples on triplet energy transfer catalysis, see
- 20aJ. Molloy, M. Schäfer, M. Wienhold, T. Morack, C. G. Daniliuc, R. Gilmour, Science 2020, 369, 302–306;
- 20bK. M. Nakafuku, Z. Zhang, E. A. Wappes, L. M. Stateman, A. D. Chen, D. A. Nagib, Nat. Chem. 2020, 12, 697–704;
- 20cM. R. Becker, E. R. Wearing, C. S. Schindler, Nat. Chem. 2020, 12, 898–905;
- 20dQ. Cheng, J. Chen, S. Lin, T. Ritter, J. Am. Chem. Soc. 2020, 142, 17287–17293;
- 20eF. M. Hörmann, C. Kerzig, T. S. Chung, A. Bauer, O. S. Wenger, T. Bach, Angew. Chem. Int. Ed. 2020, 59, 9659–9668; Angew. Chem. 2020, 132, 9746–9755;
- 20fL. Tian, N. A. Till, B. Kudisch, D. W. C. MacMillan, G. D. Scholes, J. Am. Chem. Soc. 2020, 142, 4555–4559;
- 20gM. Zhu, X.-L. Huang, H. Xu, X. Zhang, C. Zheng, S.-L. You, CCS Chem. 2021, 3, 652–664;
- 20hS. C. J. Ma, P. Bellotti, R. Guo, F. Schäfer, A. Heusler, X. Zhang, C. Daniliuc, M. K. Brown, K. N. Houk, F. Glorius, Science 2021, 371, 1338–1345;
- 20iY. Liu, D. Ni, B. G. Stevenson, V. Tripathy, S. E. Braley, K. Raghavachari, J. R. Swierk, M. K. Brown, Angew. Chem. Int. Ed. 2022, 61, e20220072; Angew. Chem. 2022, 134, e20220072.
- 21
- 21aA. Kretzschmar, C. Patze, S. T. Schwaebel, U. H. Bunz, J. Org. Chem. 2015, 80, 9126–9131;
- 21bH. E. Askey, J. D. Grayson, J. D. Tibbetts, J. C. Turner-Dore, J. M. Holmes, G. Kociok-Kohn, G. L. Wrigley, A. J. Cresswell, J. Am. Chem. Soc. 2021, 143, 15936–15945.
- 22T. O. Paulisch, L. A. Mai, F. Strieth-Kalthoff, M. J. James, C. Henkel, D. M. Guldi, F. Glorius, Angew. Chem. Int. Ed. 2022, 61, e202112695; Angew. Chem. 2022, 134, e202112695.
- 23Deposition Number 2206251 (for 6 g) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
Citing Literature
November 2, 2022
e202212201