Synergy of Weakly-Solvated Electrolyte and Optimized Interphase Enables Graphite Anode Charge at Low Temperature
Correction(s) for this article
-
Corrigendum: Synergy of Weakly-Solvated Electrolyte and Optimized Interphase Enables Graphite Anode Charge at Low Temperature
- Volume 61Issue 45Angewandte Chemie International Edition
- First Published online: October 31, 2022
Yang Yang
Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Institute of New Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Fudan University, Shanghai, 200433 China
These authors contributed equally to this work.
Search for more papers by this authorZhong Fang
Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Institute of New Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Fudan University, Shanghai, 200433 China
These authors contributed equally to this work.
Search for more papers by this authorYue Yin
Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Institute of New Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Fudan University, Shanghai, 200433 China
Search for more papers by this authorYongjie Cao
Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Institute of New Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Fudan University, Shanghai, 200433 China
Search for more papers by this authorProf. Yonggang Wang
Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Institute of New Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Fudan University, Shanghai, 200433 China
Search for more papers by this authorCorresponding Author
Prof. Xiaoli Dong
Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Institute of New Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Fudan University, Shanghai, 200433 China
Search for more papers by this authorCorresponding Author
Prof. Yongyao Xia
Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Institute of New Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Fudan University, Shanghai, 200433 China
Search for more papers by this authorYang Yang
Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Institute of New Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Fudan University, Shanghai, 200433 China
These authors contributed equally to this work.
Search for more papers by this authorZhong Fang
Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Institute of New Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Fudan University, Shanghai, 200433 China
These authors contributed equally to this work.
Search for more papers by this authorYue Yin
Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Institute of New Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Fudan University, Shanghai, 200433 China
Search for more papers by this authorYongjie Cao
Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Institute of New Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Fudan University, Shanghai, 200433 China
Search for more papers by this authorProf. Yonggang Wang
Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Institute of New Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Fudan University, Shanghai, 200433 China
Search for more papers by this authorCorresponding Author
Prof. Xiaoli Dong
Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Institute of New Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Fudan University, Shanghai, 200433 China
Search for more papers by this authorCorresponding Author
Prof. Yongyao Xia
Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Institute of New Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Fudan University, Shanghai, 200433 China
Search for more papers by this authorGraphical Abstract
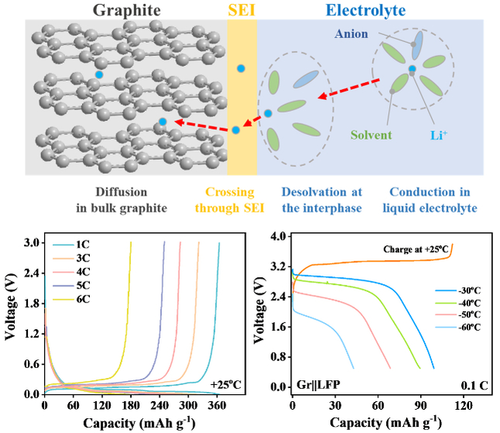
This work offers a weakly-solvated electrolyte and a high-quality SEI by room-temperature formation process, which enables graphite electrode with a fast-charging 6C-rate and low-temperature operation. Moreover, the Gr||LiFePO4 full cell exhibits a capacity retention of 78 % at −30 °C and a high discharge capacity of 37 % even at −60 °C.
Abstract
Graphite anode suffers from great capacity loss and even fails to charge (i.e. Li+-intercalation) under low temperature, mainly arising from the large overpotential including sluggish de-solvation process and insufficient ions movement in the solid electrolyte interphase (SEI). Herein, an electrolyte is developed by utilizing weakly solvated molecule ethyl trifluoroacetate and film-forming fluoroethylene carbonate to achieve smooth de-solvation and high ionic conductivity at low temperature. Evolution of SEI formed at different temperatures is further investigated to propose an effective room-temperature SEI formation strategy for low-temperature operations. The synergetic effect of tamed electrolyte and optimized SEI enables graphite with a reversible charge/discharge capacity of 183 mAh g−1 at −30 °C and fast-charging up to 6C-rate at room temperature. Moreover, graphite||LiFePO4 full cell maintains a capacity retention of 78 % at −30 °C, and 37 % even at a super-low temperature of −60 °C. This work offers a progressive insight towards fast-charging and low-temperature batteries.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202208345-sup-0001-misc_information.pdf1.3 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aW. Cai, C. Yan, Y. Yao, L. Xu, X. Chen, J. Huang, Q. Zhang, Angew. Chem. Int. Ed. 2021, 60, 13007–13012; Angew. Chem. 2021, 133, 13117–13122;
- 1bM. Weiss, R. Ruess, J. Kasnatscheew, Y. Levartovsky, N. Levy, P. Minnmann, L. Stolz, T. Waldmann, M. Mehrens, D. Aurbach, M. Winter, Y. Ein-Eli, J. Janek, Adv. Energy Mater. 2021, 11, 2101126;
- 1cX. Yang, T. Liu, Y. Gao, S. Ge, Y. Leng, D. Wang, C. Wang, Joule 2019, 3, 3002–3019.
- 2
- 2aJ. An, H. Zhang, L. Qi, G. Li, Y. Li, Angew. Chem. Int. Ed. 2022, 61, e202113313; Angew. Chem. 2022, 134, e202113313;
- 2bW. Cai, Y. Yao, G. Zhu, C. Yan, L. Jiang, C. He, J. Huang, Q. Zhang, Chem. Soc. Rev. 2020, 49, 3806–3833;
- 2cS. Zhang, K. Xu, T. Jow, J. Power Sources 2003, 115, 137–140;
- 2dS. Li, K. Wang, G. Zhang, S. Li, Y. Xu, X. Zhang, X. Zhang, S. Zheng, X. Sun, Y. Ma, Adv. Funct. Mater. 2022, 32, 2200796.
- 3
- 3aT. Gao, Y. Han, D. Fraggedakis, S. Das, T. Zhou, C.-N. Yeh, S. Xu, W. C. Chueh, J. Li, M. Z. Bazant, Joule 2021, 5, 393–414;
- 3bW. Mei, L. Jiang, C. Liang, J. Sun, Q. Wang, Energy Storage Mater. 2021, 41, 209–221;
- 3cY. Na, X. Sun, A. Fan, S. Cai, C. Zheng, Chin. Chem. Lett. 2021, 32, 973–982;
- 3dC. Yan, Y. Yao, W. Cai, L. Xu, S. Kaskel, H. Park, J. Huang, J. Energy Chem. 2020, 49, 335–338.
- 4T. Jow, S. Delp, J. Allen, J.-P. Jones, M. Smart, J. Electrochem. Soc. 2018, 165, A361–A367.
- 5
- 5aH. Cheng, Q. Sun, L. Li, Y. Zou, Y. Wang, T. Cai, F. Zhao, G. Liu, Z. Ma, W. Wahyudi, ACS Energy Lett. 2022, 7, 490–513;
- 5bQ. Dong, Y. Chu, Y. Shen, L. Chen, J. Electrochem. 2020, 26, 19–31;
- 5cS. Heiskanen, J. Kim, B. Lucht, Joule 2019, 3, 2322–2333.
- 6
- 6aN. Zhang, T. Deng, S. Zhang, C. Wang, L. Chen, C. Wang, X. Fan, Adv. Mater. 2022, 34, 2107899;
- 6bQ. Li, G. Liu, H. Cheng, Q. Sun, J. Zhang, J. Ming, Chem. Eur. J. 2021, 27, 15842–15865.
- 7
- 7aL. Xing, X. Zheng, M. Schroeder, J. Alvarado, A. von Wald Cresce, K. Xu, Q. Li, W. Li, Acc. Chem. Res. 2018, 51, 282–289;
- 7bS. Zhang, K. Xu, T. Jow, Electrochem. Commun. 2002, 4, 928–932.
- 8
- 8aJ. Holoubek, H. Liu, Z. Wu, Y. Yin, X. Xing, G. Cai, S. Yu, H. Zhou, T. A. Pascal, Z. Chen, Nat. Energy 2021, 6, 303–313;
- 8bJ. Shi, N. Ehteshami, J. Ma, H. Zhang, H. Liu, X. Zhang, J. Li, E. Paillard, J. Power Sources 2019, 429, 67–74;
- 8cX. Fan, X. Ji, L. Chen, J. Chen, T. Deng, F. Han, J. Yue, N. Piao, R. Wang, X. Zhou, X. Xiao, L. Chen, C. Wang, Nat. Energy 2019, 4, 882–890.
- 9Y. X. Yao, X. Chen, C. Yan, X. Q. Zhang, W. L. Cai, J. Q. Huang, Q. Zhang, Angew. Chem. Int. Ed. 2021, 60, 4090–4097; Angew. Chem. 2021, 133, 4136–4143.
- 10Y. Yamada, K. Furukawa, K. Sodeyama, K. Kikuchi, M. Yaegashi, Y. Tateyama, A. Yamada, J. Am. Chem. Soc. 2014, 136, 5039–5046.
- 11L. L. Jiang, C. Yan, Y. X. Yao, W. Cai, J. Q. Huang, Q. Zhang, Angew. Chem. Int. Ed. 2021, 60, 3402–3406; Angew. Chem. 2021, 133, 3444–3448.
- 12S. Tan, Z. Shadike, B. Lucht, K. Xu, C. S. Wang, X. Q. Yang, E. Y. Hu, ACS Appl. Mater. Interfaces 2021, 13, 24995–25001.
- 13
- 13aQ. Li, D. Lu, J. Zheng, S. Jiao, L. Luo, C.-M. Wang, K. Xu, J.-G. Zhang, W. Xu, ACS Appl. Mater. Interfaces 2017, 9, 42761–42768;
- 13bK. Xu, Y. Lam, S. S. Zhang, T. R. Jow, T. B. Curtis, J. Phys. Chem. C 2007, 111, 7411–7421.
- 14
- 14aY. Yang, P. Li, N. Wang, Z. Fang, C. Wang, X. Dong, Y. Xia, Chem. Commun. 2020, 56, 9640–9643;
- 14bX. Dong, Y.-G. Wang, Y. Xia, Acc. Chem. Res. 2021, 54, 3883–3894.
- 15X. Dong, Z. Guo, Z. Guo, Y. Wang, Y. Xia, Joule 2018, 2, 902–913.
- 16J. Burns, L. Krause, D.-B. Le, L. Jensen, A. Smith, D. Xiong, J. Dahn, J. Electrochem. Soc. 2011, 158, A1417–A1422.
- 17R. Petibon, C. Aiken, N. Sinha, J. Burns, H. Ye, C. M. VanElzen, G. Jain, S. Trussler, J. Dahn, J. Electrochem. Soc. 2013, 160, A117–A124.
- 18
- 18aM. Genovese, A. Louli, R. Weber, C. Martin, T. Taskovic, J. Dahn, J. Electrochem. Soc. 2019, 166, A3342–A3347;
- 18bA. Keefe, S. Buteau, I. Hill, J. Dahn, J. Electrochem. Soc. 2019, 166, A3272–A3279.
- 19
- 19aH. L. Andersen, L. Djuandhi, U. Mittal, N. Sharma, Adv. Energy Mater. 2021, 11, 2102693;
- 19bK. Edström, M. Herstedt, D. P. Abraham, J. Power Sources 2006, 153, 380–384;
- 19cE. Peled, S. Menkin, J. Electrochem. Soc. 2017, 164, A1703–A1719.
- 20
- 20aD. Wu, J. He, J. Liu, M. Wu, S. Qi, H. Wang, J. Huang, F. Li, D. Tang, J. Ma, Adv. Energy Mater. 2022, 12, 2200337;
- 20bV. Etacheri, O. Haik, Y. Goffer, G. A. Roberts, I. C. Stefan, R. Fasching, D. Aurbach, Langmuir 2012, 28, 965–976;
- 20cN. Qin, L. Jin, Y. Lu, Q. Wu, J. Zheng, C. Zhang, Z. Chen, J. Zheng, Adv. Energy Mater. 2022, 12, 2103402.
- 21
- 21aL. Chen, J. Lu, Y. Wang, P. He, S. Huang, Y. Liu, Y. Wu, G. Cao, L. Wang, X. He, J. Qiu, H. Zhang, Energy Storage Mater. 2022, 49, 493–501;
- 21bD. Aurbach, I. Weissman, A. Schechter, H. Cohen, Langmuir 1996, 12, 3991–4007;
- 21cW. Cao, J. Lu, K. Zhou, G. Sun, J. Zheng, Z. Geng, H. Li, Nano Energy 2022, 95, 106983.
- 22
- 22aG. Yang, S. Zhang, S. Weng, X. Li, X. Wang, Z. Wang, L. Chen, Nano Lett. 2021, 21, 5316–5323;
- 22bM. Lu, H. Cheng, Y. Yang, Electrochim. Acta 2008, 53, 3539–3546.