Atroposelective Construction of Nine-Membered Carbonate-Bridged Biaryls
Shiqi Jia
Green Catalysis Center, and College of Chemistry, Zhengzhou University, 100 Science Avenue, Zhengzhou, 450001 Henan, P. R. China
These authors contributed equally to this work.
Search for more papers by this authorYuhong Tian
Chongqing Key Laboratory of Natural Product Synthesis and Drug Research, Chemical Biology Research Center, School of Pharmaceutical Sciences, Chongqing University, Chongqing, 401331 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorXin Li
Chongqing Key Laboratory of Natural Product Synthesis and Drug Research, Chemical Biology Research Center, School of Pharmaceutical Sciences, Chongqing University, Chongqing, 401331 P. R. China
Search for more papers by this authorPengfei Wang
Chongqing Key Laboratory of Natural Product Synthesis and Drug Research, Chemical Biology Research Center, School of Pharmaceutical Sciences, Chongqing University, Chongqing, 401331 P. R. China
Search for more papers by this authorYu Lan
Green Catalysis Center, and College of Chemistry, Zhengzhou University, 100 Science Avenue, Zhengzhou, 450001 Henan, P. R. China
School of Chemistry and Chemical Engineering, Chongqing University, Chongqing, 401331 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Hailong Yan
Chongqing Key Laboratory of Natural Product Synthesis and Drug Research, Chemical Biology Research Center, School of Pharmaceutical Sciences, Chongqing University, Chongqing, 401331 P. R. China
Search for more papers by this authorShiqi Jia
Green Catalysis Center, and College of Chemistry, Zhengzhou University, 100 Science Avenue, Zhengzhou, 450001 Henan, P. R. China
These authors contributed equally to this work.
Search for more papers by this authorYuhong Tian
Chongqing Key Laboratory of Natural Product Synthesis and Drug Research, Chemical Biology Research Center, School of Pharmaceutical Sciences, Chongqing University, Chongqing, 401331 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorXin Li
Chongqing Key Laboratory of Natural Product Synthesis and Drug Research, Chemical Biology Research Center, School of Pharmaceutical Sciences, Chongqing University, Chongqing, 401331 P. R. China
Search for more papers by this authorPengfei Wang
Chongqing Key Laboratory of Natural Product Synthesis and Drug Research, Chemical Biology Research Center, School of Pharmaceutical Sciences, Chongqing University, Chongqing, 401331 P. R. China
Search for more papers by this authorYu Lan
Green Catalysis Center, and College of Chemistry, Zhengzhou University, 100 Science Avenue, Zhengzhou, 450001 Henan, P. R. China
School of Chemistry and Chemical Engineering, Chongqing University, Chongqing, 401331 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Hailong Yan
Chongqing Key Laboratory of Natural Product Synthesis and Drug Research, Chemical Biology Research Center, School of Pharmaceutical Sciences, Chongqing University, Chongqing, 401331 P. R. China
Search for more papers by this authorGraphical Abstract
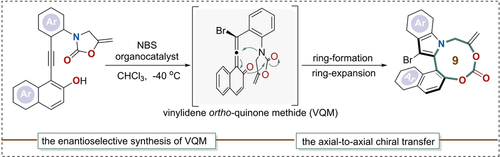
An efficient method for the atroposelective construction of nine-membered carbonate-bridged biaryls was achieved through a ring-expansion process via vinylidene ortho-quinone methide (VQM) intermediates. This strategy allows the convenient construction of bridged biaryls with broad functional group tolerance under mild conditions. In bioassay studies, several agents showed considerable antiproliferative activity via the mitochondrial-related apoptosis mechanism.
Abstract
We herein demonstrated an efficient method for the atroposelective construction of nine-membered carbonate-bridged biaryls through vinylidene ortho-quinone methide (VQM) intermediates. Diverse products with desirable pharmacological features were synthesized in satisfying yields and good to excellent enantioselectivities. In subsequent bioassays, several agents showed considerable antiproliferative activity via the mitochondrial-related apoptosis mechanism. Further transformations produced more structural diversity and may inspire new ideas for developing functional molecules.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202206501-sup-0001-1c.cif270.5 KB | Supporting Information |
| anie202206501-sup-0001-1m.cif527.9 KB | Supporting Information |
| anie202206501-sup-0001-2w.cif789.4 KB | Supporting Information |
| anie202206501-sup-0001-2y.cif974 KB | Supporting Information |
| anie202206501-sup-0001-4a.cif634.9 KB | Supporting Information |
| anie202206501-sup-0001-6a.cif479.6 KB | Supporting Information |
| anie202206501-sup-0001-misc_information.pdf12.8 MB | Supporting Information |
| anie202206501-sup-0001-R-2K.cif289 KB | Supporting Information |
| anie202206501-sup-0001-S-2k.cif205.1 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. C. Kozlowski, B. J. Morgan, E. C. Linton, Chem. Soc. Rev. 2009, 38, 3193–3207;
- 1bS. R. Laplante, L. D. Fader, K. R. Fandrick, D. R. Fandrick, O. Hucke, R. Kemper, S. P. F. Miller, P. J. Edwards, J. Med. Chem. 2011, 54, 7005–7022;
- 1cD. Zhang, Q. Wang, Coord. Chem. Rev. 2015, 286, 1–16;
- 1dE. Kumarasamy, R. Raghunathan, M. P. Sibi, J. Sivaguru, Chem. Rev. 2015, 115, 11239–11300.
- 2
- 2aM. Penhoat, V. Levacher, G. Dupas, J. Org. Chem. 2003, 68, 9517–9520;
- 2bG. Bringmann, A. J. P. Mortimer, P. A. Keller, M. J. Gresser, J. Garner, M. Breuning, Angew. Chem. Int. Ed. 2005, 44, 5384–5427; Angew. Chem. 2005, 117, 5518–5563;
- 2cX. Su, G. L. Thomas, W. R. J. D. Galloway, D. S. Surry, R. J. Spandl, D. R. Spring, Synthesis 2009, 3880–3896;
- 2dG. Bringmann, T. Gulder, T. A. M. Gulder, M. Breuning, Chem. Rev. 2011, 111, 563–639.
- 3
- 3aA. Urbano, A. Urbano, Angew. Chem. Int. Ed. 2003, 42, 3986–3989; Angew. Chem. 2003, 115, 4116–4119;
- 3bY. Shen, C. Chen, Chem. Rev. 2012, 112, 1463–1535;;
- 3cM. Gingras, Chem. Soc. Rev. 2013, 42, 968–1006;
- 3dA. Urbano, M. C. Carreño, Org. Biomol. Chem. 2013, 11, 699–708;
- 3eK. Tanaka, Y. Kimura, K. Murayama, Bull. Chem. Soc. Jpn. 2015, 88, 375–385;
- 3fI. G. Stará, I. Starý, Acc. Chem. Res. 2020, 53, 144–158.
- 4
- 4aF. K. Brown, J. C. Hempel, J. S. Dixon, S. Amato, L. Mueller, P. W. Jeffs, J. Am. Chem. Soc. 1989, 111, 7328–7333;
- 4bS. Gladiali, A. Dore, D. Fabbri, O. de Lucchi, G. Valle, J. Org. Chem. 1994, 59, 6363–6371;
- 4cT. B. Freedman, X. Cao, L. A. Nafie, M. Kalbermatter, A. Linden, A. J. Rippert, Helv. Chim. Acta 2003, 86, 3141–3155.
- 5
- 5aG. A. Molander, Acc. Chem. Res. 1998, 31, 603–609;
- 5bS. K. Chattopadhyay, S. Karmakar, T. Biswas, K. C. Majumdar, H. Rahaman, B. Roy, Tetrahedron 2007, 63, 3919–3952;
- 5cI. Shiina, Chem. Rev. 2007, 107, 239–273;
- 5dK. C. Majumdar, RSC Adv. 2011, 1, 1152–1170;
- 5eA. Hussain, S. K. Yousuf, D. Mukherjee, RSC Adv. 2014, 4, 43241–43257.
- 6
- 6aY. Bin Wang, B. Tan, Acc. Chem. Res. 2018, 51, 534–547;
- 6bB. Da, S. Xiang, S. Li, B. Tan, Chin. J. Chem. 2021, 39, 1787–1796;
- 6cJ. K. Cheng, S. H. Xiang, S. Li, L. Ye, B. Tan, Chem. Rev. 2021, 121, 4805–4902;
- 6dL. W. Qi, S. Li, S. H. Xiang, J. (Joelle) Wang, B. Tan, Nat. Catal. 2019, 2, 314–323;
- 6eS. Yan, W. Xia, S. Li, Q. Song, S. H. Xiang, B. Tan, J. Am. Chem. Soc. 2020, 142, 7322–7327;
- 6fS. Wu, S. H. Xiang, S. Li, W. Y. Ding, L. Zhang, P. Y. Jiang, Z. A. Zhou, B. Tan, Nat. Catal. 2021, 4, 692–702.
- 7
- 7aB. Greve, P. Imming, J. Org. Chem. 1997, 62, 8058–8062;
- 7bR. L. Reyes, T. Iwai, M. Sawamura, Chem. Rev. 2021, 121, 8926–8947.
- 8
- 8aM. Wang, Z. Huang, J. Xu, Y. R. Chi, J. Am. Chem. Soc. 2014, 136, 1214–1217;
- 8bM. Wang, Z. Q. Rong, Y. Zhao, Chem. Commun. 2014, 50, 15309–15312;
- 8cX. Wu, L. Zhou, R. Maiti, C. Mou, L. Pan, Y. R. Chi, Angew. Chem. Int. Ed. 2019, 58, 477–481; Angew. Chem. 2019, 131, 487–491;
- 8dG. Bertuzzi, M. K. Thøgersen, M. Giardinetti, A. Vidal-Albalat, A. Simon, K. N. Houk, K. A. Jørgensen, J. Am. Chem. Soc. 2019, 141, 3288–3297;
- 8eW. Liang, K. Jiang, F. Du, J. Yang, L. Shuai, Q. Ouyang, Y. C. Chen, Y. Wei, Angew. Chem. Int. Ed. 2020, 59, 19222–19228; Angew. Chem. 2020, 132, 19384–19390;
- 8fY. Gao, X. Song, R. J. Yan, W. Du, Y. C. Chen, Org. Biomol. Chem. 2021, 19, 151–155;
- 8gZ. Chen, Z.-C. Chen, W. Du, Y.-C. Chen, Org. Lett. 2021, 23, 8559–8564.
- 9
- 9aD. McLeod, A. Cherubini-Celli, N. Sivasothirajah, C. H. McCulley, M. L. Christensen, K. A. Jørgensen, Chem. Eur. J. 2020, 26, 11417–11422;
- 9bB. Jiang, W. Du, Y. C. Chen, Chem. Commun. 2020, 56, 7257–7260.
- 10
- 10aL. C. Yang, Z. Q. Rong, Y. N. Wang, Z. Y. Tan, M. Wang, Y. Zhao, Angew. Chem. Int. Ed. 2017, 56, 2927–2931; Angew. Chem. 2017, 129, 2973–2977;
- 10bP. Yu, C. Q. He, A. Simon, W. Li, R. Mose, M. K. Thøgersen, K. A. Jørgensen, K. N. Houk, J. Am. Chem. Soc. 2018, 140, 13726–13735;
- 10cG. Yang, Y. M. Ke, Y. Zhao, Angew. Chem. Int. Ed. 2021, 60, 12775–12780; Angew. Chem. 2021, 133, 12885–12890.
- 11Y. N. Wang, L. C. Yang, Z. Q. Rong, T. L. Liu, R. Liu, Y. Zhao, Angew. Chem. Int. Ed. 2018, 57, 1596–1600; Angew. Chem. 2018, 130, 1612–1616.
- 12
- 12aT. Saget, N. Cramer, Angew. Chem. Int. Ed. 2013, 52, 7865–7868; Angew. Chem. 2013, 125, 8019–8022;
- 12bJ. Liu, X. Yang, Z. Zuo, J. Nan, Y. Wang, X. Luan, Org. Lett. 2018, 20, 244–247;
- 12cC. G. Newton, E. Braconi, J. Kuziola, M. D. Wodrich, N. Cramer, Angew. Chem. Int. Ed. 2018, 57, 11040–11044; Angew. Chem. 2018, 130, 11206–11210;
- 12dX. Xue, Z. Gu, Org. Lett. 2019, 21, 3942–3945;
- 12eY. Zhang, Y. Q. Liu, L. Hu, X. Zhang, Q. Yin, Org. Lett. 2020, 22, 6479–6483.
- 13S. Lu, J. Y. Ong, H. Yang, S. B. Poh, X. Liew, C. S. D. Seow, M. W. Wong, Y. Zhao, J. Am. Chem. Soc. 2019, 141, 17062–17067.
- 14
- 14aA. R. Khan, J. C. Parrish, M. E. Fraser, W. W. Smith, P. A. Bartlett, M. N. G. James, Biochemistry 1998, 37, 16839–16845;
- 14bT. Rezai, B. Yu, G. L. Millhauser, M. P. Jacobson, R. S. Lokey, J. Am. Chem. Soc. 2006, 128, 2510–2511;
- 14cY. U. Kwon, T. Kodadek, Chem. Biol. 2007, 14, 671–677;
- 14dR. D. Taylor, M. Maccoss, A. D. G. Lawson, J. Med. Chem. 2014, 57, 5845–5859.
- 15
- 15aW. Chen, Y. Han, X. Peng, Chem. Eur. J. 2014, 20, 7410–7418;
- 15bV. Janganati, J. Ponder, C. T. Jordan, M. J. Borrelli, N. R. Penthala, P. A. Crooks, J. Med. Chem. 2015, 58, 8896–8906;
- 15cW. S. Shin, J. Han, P. Verwilst, R. Kumar, J. H. Kim, J. S. Kim, Bioconjugate Chem. 2016, 27, 1419–1426;
- 15dC. Jin, S. Wen, Q. Zhang, Q. Zhu, J. Yu, W. Lu, ACS Med. Chem. Lett. 2017, 8, 762–765.
- 16
- 16aS. Arae, M. Furusawa, S. Beppu, K. Igawa, K. Tomooka, R. Irie, Chimia 2018, 72, 892–899;
- 16bJ. Rodriguez, D. Bonne, Chem. Commun. 2019, 55, 11168–11170;
- 16cF. Doria, C. Percivalle, M. Freccero, J. Org. Chem. 2012, 77, 3615–3619;
- 16dX. Wu, L. Xue, D. Li, S. Jia, J. Ao, J. Deng, H. Yan, Angew. Chem. Int. Ed. 2017, 56, 13722–13726; Angew. Chem. 2017, 129, 13910–13914;
- 16eY. Liu, X. Wu, S. Li, L. Xue, C. Shan, Z. Zhao, H. Yan, Angew. Chem. Int. Ed. 2018, 57, 6491–6495; Angew. Chem. 2018, 130, 6601–6605;
- 16fL. Peng, K. Li, C. Xie, S. Li, D. Xu, W. Qin, H. Yan, Angew. Chem. Int. Ed. 2019, 58, 17199–17204; Angew. Chem. 2019, 131, 17359–17364;
- 16gS. Jia, Z. Chen, N. Zhang, Y. Tan, Y. Liu, J. Deng, H. Yan, J. Am. Chem. Soc. 2018, 140, 7056–7060;
- 16hY. Tan, S. Jia, F. Hu, Y. Liu, L. Peng, D. Li, H. Yan, J. Am. Chem. Soc. 2018, 140, 16893–16898;
- 16iS. Jia, S. Li, Y. Liu, W. Qin, H. Yan, Angew. Chem. Int. Ed. 2019, 58, 18496–18501; Angew. Chem. 2019, 131, 18667–18672;
- 16jS. Huang, H. Wen, Y. Tian, P. Wang, W. Qin, H. Yan, Angew. Chem. Int. Ed. 2021, 60, 21486–21493; Angew. Chem. 2021, 133, 21656–21663;
- 16kD. Xu, S. Huang, F. Hu, L. Peng, S. Jia, H. Mao, X. Gong, F. Li, W. Qin, H. Yan, CCS Chem. 2021, 3, 2680–2691;
- 16lY.-B. Wang, P. Yu, Z.-P. Zhou, J. Zhang, J. Wang, S.-H. Luo, Q.-S. Gu, K. N. Houk, B. Tan, Nat. Catal. 2019, 2, 504–513;
- 16mL. Zhang, J. Shen, S. Wu, G. Zhong, Y.-B. Wang, B. Tan, Angew. Chem. Int. Ed. 2020, 59, 23077–23082; Angew. Chem. 2020, 132, 23277–23282;
- 16nH. Liu, K. Li, S. Huang, H. Yan, Angew. Chem. Int. Ed. 2022, 61, e202117063; Angew. Chem. 2022, 134, e202117063;
- 16oK. Li, S. Huang, T. Liu, S. Jia, H. Yan, J. Am. Chem. Soc. 2022, 144, 7374–7381;
- 16pY. Chang, C. Xie, H. Liu, S. Huang, P. Wang, W. Qin, H. Yan, Nat. Commun. 2022, 13, 1933–1943.
- 17
- 17aT. Sarkar, B. K. Das, K. Talukdar, T. A. Shah, T. Punniyamurthy, ACS Omega 2020, 5, 26316–26328;
- 17bA. K. Clarke, W. P. Unsworth, Chem. Sci. 2020, 11, 2876–2881;
- 17cP. Natho, L. A. T. Allen, P. J. Parsons, Tetrahedron Lett. 2020, 61, 151695;
- 17dB. Biletskyi, P. Colonna, K. Masson, J. L. Parrain, L. Commeiras, G. Chouraqui, Chem. Soc. Rev. 2021, 50, 7513–7538.
- 18
- 18aS.-K. Tian, Y. Chen, J. Hang, L. Tang, P. McDaid, L. Deng, Acc. Chem. Res. 2004, 37, 621–631;
- 18bA. G. Doyle, E. N. Jacobsen, Chem. Rev. 2007, 107, 5713–5743;
- 18cD. W. C. MacMillan, Nature 2008, 455, 304–308;
- 18d Cinchona Alkaloids in Synthesis and Catalysis, Ligands, Immobilization and Organocatalysis (Ed.: C. E. Song), Wiley-VCH, Weinheim, 2009;
10.1002/9783527628179 Google Scholar
- 18eQ. Zhu, Y. Lu, Angew. Chem. Int. Ed. 2010, 49, 7753–7756; Angew. Chem. 2010, 122, 7919–7922;
- 18fH. X. He, D. M. Du, Eur. J. Org. Chem. 2014, 6190–6199.
- 19Deposition Numbers 2168090 (for 1c), 2167666 (for 1m), 2115930 (for 2k), 2120077 (for 2s), 2120076 (for 2w), 2115927 (for 2y), 2120078 (for 4a), 2120079 (for 6a) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 20
- 20aS. Sarel, I. Levin, L. A. Pohoryles, J. Chem. Soc. 1960, 3079–3082;
- 20bB. Alcaide, P. Almendros, M. T. Quirós, R. López, M. I. Menéndez, A. Sochacka-Ćwikła, J. Am. Chem. Soc. 2013, 135, 898–905.
- 21
- 21aZ. S. Liu, P. P. Xie, Y. Hua, C. Wu, Y. Ma, J. Chen, H. G. Cheng, X. Hong, Q. Zhou, Chem 2021, 7, 1917–1932;
- 21bQ. Gao, C. Wu, S. Deng, L. Li, Z. S. Liu, Y. Hua, J. Ye, C. Liu, H. G. Cheng, H. Cong, Y. Jiao, Q. Zhou, J. Am. Chem. Soc. 2021, 143, 7253–7260;
- 21cZ. S. Liu, Y. Hua, Q. Gao, Y. Ma, H. Tang, Y. Shang, H. G. Cheng, Q. Zhou, Nat. Catal. 2020, 3, 727–733.
- 22
- 22aJ.-J. Wang, Y.-K. Shen, W.-P. Hu, M.-C. Hsieh, F.-L. Lin, M.-K. Hsu, M.-H. Hsu, J. Med. Chem. 2006, 49, 1442–1449;
- 22bY. Yamamoto, M. Kurazono, Bioorg. Med. Chem. Lett. 2007, 17, 1626–1628;
- 22cA. S. Gurkan-Alp, M. Mumcuoglu, C. A. Andac, E. Dayanc, R. Cetin-Atalay, E. Buyukbingol, Eur. J. Med. Chem. 2012, 58, 346–354;
- 22dS.-H. Zhuang, Y.-C. Lin, L.-C. Chou, M.-H. Hsu, H.-Y. Lin, C.-H. Huang, J.-C. Lien, S.-C. Kuo, L.-J. Huang, Eur. J. Med. Chem. 2013, 66, 466–479;
- 22eD.-J. Hwang, J. Wang, W. Li, D. D. Miller, ACS Med. Chem. Lett. 2015, 6, 993–997;
- 22fC. Sherer, T. J. Snape, Eur. J. Med. Chem. 2015, 97, 552–560;
- 22gM.-Z. Zhang, C.-Y. Jia, Y.-C. Gu, N. Mulholland, S. Turner, D. Beattie, W.-H. Zhang, G.-F. Yang, J. Clough, Eur. J. Med. Chem. 2017, 126, 669–674.