Formation of a Hybrid 1-Bora-3-boratabenzene Heteroarene Anion Derivative
Dr. Qiu Sun
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstraße 40, 48149 Münster, Germany
Search for more papers by this authorDr. Constantin G. Daniliuc
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstraße 40, 48149 Münster, Germany
Search for more papers by this authorDr. Christian Mück-Lichtenfeld
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstraße 40, 48149 Münster, Germany
Search for more papers by this authorDr. Gerald Kehr
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstraße 40, 48149 Münster, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Gerhard Erker
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstraße 40, 48149 Münster, Germany
Search for more papers by this authorDr. Qiu Sun
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstraße 40, 48149 Münster, Germany
Search for more papers by this authorDr. Constantin G. Daniliuc
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstraße 40, 48149 Münster, Germany
Search for more papers by this authorDr. Christian Mück-Lichtenfeld
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstraße 40, 48149 Münster, Germany
Search for more papers by this authorDr. Gerald Kehr
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstraße 40, 48149 Münster, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Gerhard Erker
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstraße 40, 48149 Münster, Germany
Search for more papers by this authorGraphical Abstract
Abstract
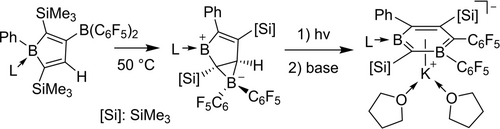
The B(C6F5)2-substituted borole 2⋅SMe2 was obtained from the phenyl bis(trimethylsilylethynyl) borane SMe2 adduct (1⋅SMe2) by a synthetic sequence containing a rare 1,1-hydroboration reaction. Subsequent thermolysis at 50 °C converted it to the bicyclic borenium/borate zwitterion 3⋅SMe2. Photolysis of 3⋅SMe2 gave the diboracyclohexadiene derivative 4⋅SMe2, which after ligand exchange with N,N-dimesitylimidazolylidene and deprotonation gave the 1-bora-3-boratabenzene anion derivative as its potassium salt 6. Some unique follow-up reactions of the unsaturated diboron containing six-membered heterocycles were investigated.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202205565-sup-0001-all.cif16.5 MB | Supporting Information |
| anie202205565-sup-0001-checkcif_all.pdf552.5 KB | Supporting Information |
| anie202205565-sup-0001-misc_information.pdf4.7 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. Waluk, H.-P. Klein, A. J. Ashe, J. Michl, Organometallics 1989, 8, 2804–2808;
- 1bC. Batich, E. Heilbronner, V. Hornung, A. J. Ashe, D. T. Clark, U. T. Cobley, D. Kilcast, I. Scanlan, J. Am. Chem. Soc. 1973, 95, 928–930.
- 2
- 2aD. L. Thorn, R. Hoffmann, Nouv. J. Chim. 1979, 3, 39–45;
- 2bG. P. Elliott, W. R. Roper, J. M. Waters, J. Chem. Soc. Chem. Commun. 1982, 811–813;
- 2cJ. R. Bleeke, Y.-F. Xie, W.-J. Peng, M. Chiang, J. Am. Chem. Soc. 1989, 111, 4118–4120;
- 2dJ. R. Bleeke, Acc. Chem. Res. 1991, 24, 271–277;
- 2eD. Chen, Y. Hua, H. Xia, Chem. Rev. 2020, 120, 12994–13086.
- 3
- 3aG. H. Herberich, G. Greiss, H. F. Heil, Angew. Chem. Int. Ed. Engl. 1970, 9, 805–806; Angew. Chem. 1970, 82, 838–839;
- 3bG. H. Herberich, H. Ohst, Adv. Organomet. Chem. 1986, 25, 199–236.
- 4A. J. Ashe, P. Shu, J. Am. Chem. Soc. 1971, 93, 1804–1805.
- 5
- 5aR. Boese, N. Finke, J. Henkelmann, G. Maier, P. Paetzold, H. P. Reisenauer, G. Schmid, Chem. Ber. 1985, 118, 1644–1654;
- 5bR. Boese, N. Finke, T. Keil, P. Paetzold, G. Schmid, Z. Naturforsch. B 1985, 40, 1327–1332;
- 5cD. A. Hoic, W. M. Davis, G. C. Fu, J. Am. Chem. Soc. 1995, 117, 8480–8481;
- 5dD. A. Hoic, J. R. Wolf, W. M. Davis, G. C. Fu, Organometallics 1996, 15, 1315–1318;
- 5eS. Qiao, D. A. Hoic, G. C. Fu, J. Am. Chem. Soc. 1996, 118, 6329–6330;
- 5fD. A. Hoic, M. DiMare, G. C. Fu, J. Am. Chem. Soc. 1997, 119, 7155–7156;
- 5gS. Qiao, D. A. Hoic, G. C. Fu, Organometallics 1997, 16, 1501–1502;
- 5hG. C. Fu, Adv. Organomet. Chem. 2001, 47, 101–119;
- 5iC.-T. Shen, Y.-H. Liu, S.-M. Peng, C.-W. Chiu, Angew. Chem. Int. Ed. 2013, 52, 13293–13297; Angew. Chem. 2013, 125, 13535–13539; for a general review on boron containing aromatic heterocycles see e.g.:
- 5jB. Su, R. Kinjo, Synthesis 2017, 49, 2985–3034.
- 6M. Arrowsmith, J. Bçhnke, H. Braunschweig, M. A. Celik, C. Claes, W. C. Ewing, I. Krummenacher, K. Lubitz, C. Schneider, Angew. Chem. Int. Ed. 2016, 55, 11271–11275; Angew. Chem. 2016, 128, 11441–11445.
- 7For selected 1,2-diboratabenzene derivatives examples see:
- 7aG. E. Herberich, B. Hessner, M. Hostalek, Angew. Chem. Int. Ed. Engl. 1986, 25, 642–643; Angew. Chem. 1986, 98, 637–638;
- 7bG. E. Herberich, B. Hessner, M. Hostalek, J. Organomet. Chem. 1988, 355, 473–484;
- 7cW. Weinmann, H. Pritzkow, W. Siebert, Chem. Ber. 1994, 127, 611–613;
- 7dA. Wakamiya, K. Mori, T. Araki, S. Yamaguchi, J. Am. Chem. Soc. 2009, 131, 10850–10851;
- 7eT. Araki, A. Wakamiya, K. Mori, S. Yamaguchi, Chem. Asian J. 2012, 7, 1594–1603.
- 8For selected 1,3-diboratabenzene derivatives examples see:
- 8aG. E. Herberich, H. Ohst, H. Mayer, Angew. Chem. Int. Ed. Engl. 1984, 23, 969–970; Angew. Chem. 1984, 96, 975–976;
- 8bG. E. Herberich, H. Ohst, J. Organomet. Chem. 1986, 307, C16–C18;
- 8cG. E. Herberich, B. Hessner, H. Ohst, J. Organomet. Chem. 1989, 375, 161–166;
- 8dC. Balzereit, H.-J. Winkler, W. Massa, A. Berndt, Angew. Chem. Int. Ed. Engl. 1994, 33, 2306–2308; Angew. Chem. 1994, 106, 2394–2396;
- 8eB. Deobald, J. Hauss, H. Pritzkow, D. Steiner, A. Bemdt, W. Siebert, J. Organomet. Chem. 1994, 481, 205–210.
- 9For selected 1,4-diboratabenzene derivatives and related compounds see:
- 9aG. E. Herberich, B. Heher, Chem. Ber. 1982, 115, 3115–3127;
- 9bP. Müller, H. Pritzkow, W. Siebert, J. Organomet. Chem. 1996, 524, 41–47;
- 9cA. Lorbach, M. Bolte, H.-W. Lerner, M. Wagner, Organometallics 2010, 29, 5762–5765;
- 9dE. von Grotthuss, M. Diefenbach, M. Bolte, H.-W. Lerner, M. C. Holthausen, M. Wagner, Angew. Chem. Int. Ed. 2016, 55, 14067–14071; Angew. Chem. 2016, 128, 14273–14277;
- 9eJ. W. Taylor, A. McSkimming, C. F. Guzman, W. H. Harman, J. Am. Chem. Soc. 2017, 139, 11032–11035;
- 9fE. von Grotthuss, S. E. Prey, M. Bolte, H.-W. Lerner, M. Wagner, J. Am. Chem. Soc. 2019, 141, 6082–6091.
- 10
- 10aD. J. Parks, W. E. Piers, G. P. A. Yap, Organometallics 1998, 17, 5492–5503;
- 10bE. A. Patrick, W. E. Piers, Chem. Commun. 2020, 56, 841–852;
- 10cW. E. Piers, T. Chivers, Chem. Soc. Rev. 1997, 26, 345–354.
- 11
- 11aB. Wrackmeyer, C. Bihlmayer, J. Chem. Soc. Chem. Commun. 1981, 1093–1094;
- 11bR. L. Ernest, W. Quinatana, R. Rosen, P. J. Carroll, L. G. Sneddon, Organometallics 1987, 6, 80–88;
- 11cP. Paetzold, E. Leuschner, Z. Anorg. Allg. Chem. 2002, 628, 658–660;
- 11dQ. Feng, H. Wu, X. Li, L. Song, L. W. Chung, Y.-D. Wu, J. Sun, J. Am. Chem. Soc. 2020, 142, 13867–13877;
- 11eA. Ueno, X. Tao, C. G. Daniliuc, G. Kehr, G. Erker, Organometallics 2018, 37, 2665–2668;
- 11fK. Škoch, C. G. Daniliuc, G. Kehr, G. Erker, Angew. Chem. Int. Ed. 2021, 60, 6757–6763; Angew. Chem. 2021, 133, 6831–6837.
- 12
- 12aA. Sebald, B. Wrackmeyer, J. Organomet. Chem. 1986, 307, 157–165;
- 12bB. Wrackmeyer, Heteroat. Chem. 2006, 17, 188–208.
- 13F. Ge, G. Kehr, C. G. Daniliuc, G. Erker, J. Am. Chem. Soc. 2014, 136, 68–71.
- 14See a rare example of this framework type for a comparison example: ref. [8d].
- 15
- 15aP. K. Prolle, H. Nöth, Chem. Rev. 1985, 85, 399–418;
10.1021/cr00069a004 Google Scholar
- 15bW. E. Piers, S. C. Bourke, K. D. Conroy, Angew. Chem. Int. Ed. 2005, 44, 5016–5036; Angew. Chem. 2005, 117, 5142–5163;
- 15cP. Eisenberger, C. M. Crudden, Dalton Trans. 2017, 46, 4874–4887;
- 15dD. Franz, S. Inoue, Chem. Eur. J. 2019, 25, 2898–2926.
- 16
- 16aG. Gabbert, W. Weinmann, H. Pritzkow, W. Siebert, Angew. Chem. Int. Ed. Engl. 1992, 31, 1603–1605; Angew. Chem. 1992, 104, 1670–1672;
- 16bC. Chen, C. G. Daniliuc, G. Kehr, G. Erker, J. Am. Chem. Soc. 2021, 143, 21312–21320.
- 17See examples of boraalkene structure for comparison:
- 17aA. Berndt, Angew. Chem. Int. Ed. Engl. 1993, 32, 985–1009; Angew. Chem. 1993, 105, 1034–1058;
- 17bP. P. Power, Chem. Rev. 1999, 99, 3463–3503;
- 17cR. C. Fischer, P. P. Power, Chem. Rev. 2010, 110, 3877–3923;
- 17dR. Borthakur, V. Chandrasekhar, Coord. Chem. Rev. 2021, 429, 213647;
- 17eM. Devillard, G. Alcaraz in PATAI'S Chemistry of Functional Groups (Ed.: Z. Rappoport), Wiley-VCH, Weinheim, 2021,
https://doi.org/10.1002/9780470682531.pat0972.
10.1002/9780470682531.pat0972 Google Scholar
- 18See examples of borataalkene structure for comparison:
- 18aM. W. Rathke, R. Kow, J. Am. Chem. Soc. 1972, 94, 6854–6856;
- 18bR. Kow, M. W. Rathke, J. Am. Chem. Soc. 1973, 95, 2715–2716;
- 18cM. M. Olmstead, P. P. Power, K. J. Weese, J. Am. Chem. Soc. 1987, 109, 2541–2542;
- 18dC.-W. Chiu, F. P. Gabbaï, Angew. Chem. Int. Ed. 2007, 46, 6878–6881; Angew. Chem. 2007, 119, 7002–7005;
- 18eK. Watanabe, A. Ueno, X. Tao, K. Škoch, X. Jie, S. Vagin, B. Rieger, C. G. Daniliuc, M. Letzel, G. Kehr, G. Erker, Chem. Sci. 2020, 11, 7349–7355;
- 18fN. A. Phillips, R. Y. Kong, A. J. P. White, M. R. Crimmin, Angew. Chem. Int. Ed. 2021, 60, 12013–12019; Angew. Chem. 2021, 133, 12120–12126.
- 19
- 19aP. V. R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao, N. J. R. V. E. Hommes, J. Am. Chem. Soc. 1996, 118, 6317–6318;
- 19bZ. Chen, C. S. Wannere, C. Corminboeuf, R. Puchta, P. V. R. Schleyer, Chem. Rev. 2005, 105, 3842–3888.
- 20See for a comparison:
- 20aJ. Böhnke, H. Braunschweig, J. O. C. Jiménez-Halla, I. Krummenacher, T. E. Stennett, J. Am. Chem. Soc. 2018, 140, 848–853;
- 20bR. Báez-Grez, R. Pino-Rios, New J. Chem. 2020, 44, 18069–18073;
- 20cJ. Poater, C. Vinas, I. Bennour, S. Escayola, M. Solà, F. Teixidor, J. Am. Chem. Soc. 2020, 142, 9396–9407.
- 21See examples of neutral nido-tetracarbahexaborane(6) for a comparison:
- 21aT. P. Onak, G. T. F. Wong, J. Am. Chem. Soc. 1970, 92, 5226;
- 21bV. R. Miller, R. N. Grimes, Inorg. Chem. 1972, 11, 862–865;
- 21cL. Killian, B. Wrackmeyer, J. Organomet. Chem. 1977, 132, 213–221;
- 21dH.-O. Berger, H. Nöth, B. Wrackmeyer, Chem. Ber. 1979, 112, 2884–2893;
- 21eH. Braunschweig, S. Ghosh, J. O. Jimenez-Halla, J. H. Klein, C. Lambert, K. Radacki, A. Steffen, A. Vargas, J. Wahler, Chem. Eur. J. 2015, 21, 210–218;
- 21fY. Canac, G. Bertrand, Angew. Chem. Int. Ed. 2003, 42, 3578–3580; Angew. Chem. 2003, 115, 3702–3704.
- 22See for a comparison:
- 22aB. Wang, Y. Li, R. Ganguly, R. D. Webster, R. Kinjo, Angew. Chem. Int. Ed. 2018, 57, 7826–7829; Angew. Chem. 2018, 130, 7952–7955;
- 22bB. Wrackmeyer, G. Kehr, Polyhedron 1991, 10, 1497–1506;
- 22cB. Wrackmeyer, G. Kehr, J. Organomet. Chem. 1995, 501, 87–93.
- 23For an example of a remotely related rupture of the carbon monoxide C≡O bond see: Q. Sun, C. G. Daniliuc, C. Mück-Lichtenfeld, K. Bergander, G. Kehr, G. Erker, J. Am. Chem. Soc. 2020, 142, 17260–17264.
- 24For examples of isonitrile in C−C coupling reactions see, for example:
- 24aJ. Li, C. G. Daniliuc, C. Mück-Lichtenfeld, G. Kehr, G. Erker, Angew. Chem. Int. Ed. 2019, 58, 15377–15380; Angew. Chem. 2019, 131, 15521–15524;
- 24bJ. Li, C. G. Daniliuc, K. K. Kartha, G. Fernández, G. Kehr, G. Erker, J. Am. Chem. Soc. 2021, 143, 2059–2067; for reviews see:
- 24cE. Schwartz, M. Koepf, H. J. Kitto, R. J. M. Nolte, A. E. Rowan, Polym. Chem. 2011, 2, 33–47;
- 24dT. Nakano, Y. Okamoto, Chem. Rev. 2001, 101, 4013–4038.
- 25B. Wang, K. Koshino, R. Kinjo, Chem. Commun. 2019, 55, 13012–13014.
- 26Deposition Numbers 2115963 (for 2⋅SMe2), 2158103 (for 3⋅SMe2), 2158104 (4⋅IMes), 2158105 (Ci-5-trans), 2158106 (6), 2158107 (8), 2164760 (9), and 2158108 (16) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.