Catalytic Atroposelective Electrophilic Amination of Indoles
Jingyang Qin
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology (HUST), Luoyu Road 1037, Wuhan, 430074 China
These authors contributed equally to this work.
Search for more papers by this authorTong Zhou
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology (HUST), Luoyu Road 1037, Wuhan, 430074 China
These authors contributed equally to this work.
Search for more papers by this authorTai-Ping Zhou
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology (HUST), Luoyu Road 1037, Wuhan, 430074 China
These authors contributed equally to this work.
Search for more papers by this authorLangyu Tang
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology (HUST), Luoyu Road 1037, Wuhan, 430074 China
Search for more papers by this authorHonghua Zuo
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology (HUST), Luoyu Road 1037, Wuhan, 430074 China
Search for more papers by this authorHuaibin Yu
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology (HUST), Luoyu Road 1037, Wuhan, 430074 China
Search for more papers by this authorProf. Guojiao Wu
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology (HUST), Luoyu Road 1037, Wuhan, 430074 China
Search for more papers by this authorProf. Yuzhou Wu
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology (HUST), Luoyu Road 1037, Wuhan, 430074 China
Search for more papers by this authorCorresponding Author
Prof. Rong-Zhen Liao
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology (HUST), Luoyu Road 1037, Wuhan, 430074 China
Search for more papers by this authorCorresponding Author
Prof. Fangrui Zhong
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology (HUST), Luoyu Road 1037, Wuhan, 430074 China
Search for more papers by this authorJingyang Qin
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology (HUST), Luoyu Road 1037, Wuhan, 430074 China
These authors contributed equally to this work.
Search for more papers by this authorTong Zhou
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology (HUST), Luoyu Road 1037, Wuhan, 430074 China
These authors contributed equally to this work.
Search for more papers by this authorTai-Ping Zhou
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology (HUST), Luoyu Road 1037, Wuhan, 430074 China
These authors contributed equally to this work.
Search for more papers by this authorLangyu Tang
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology (HUST), Luoyu Road 1037, Wuhan, 430074 China
Search for more papers by this authorHonghua Zuo
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology (HUST), Luoyu Road 1037, Wuhan, 430074 China
Search for more papers by this authorHuaibin Yu
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology (HUST), Luoyu Road 1037, Wuhan, 430074 China
Search for more papers by this authorProf. Guojiao Wu
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology (HUST), Luoyu Road 1037, Wuhan, 430074 China
Search for more papers by this authorProf. Yuzhou Wu
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology (HUST), Luoyu Road 1037, Wuhan, 430074 China
Search for more papers by this authorCorresponding Author
Prof. Rong-Zhen Liao
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology (HUST), Luoyu Road 1037, Wuhan, 430074 China
Search for more papers by this authorCorresponding Author
Prof. Fangrui Zhong
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology (HUST), Luoyu Road 1037, Wuhan, 430074 China
Search for more papers by this authorGraphical Abstract
Abstract
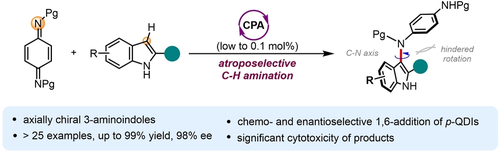
Reported here is the first catalytic atroposelective electrophilic amination of indoles, which delivers functionalized atropochiral N-sulfonyl-3-arylaminoindoles with excellent optical purity. This reaction was furnished by 1,6-nucleophilic addition to p-quinone diimines. Control experiments suggest an ionic mechanism that differs from the radical addition pathway commonly proposed for 1,6-addition to quinones. The origin of 1,6-addition selectivity was investigated through computational studies. Preliminary studies show that the obtained 3-aminoindoles atropisomers exhibit anticancer activities. This method is valuable with respect to enlarging the toolbox for atropochiral amine derivatives.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202205159-sup-0001-20191227_QJY.cif1.3 MB | Supporting Information |
| anie202205159-sup-0001-checkcif.pdf138.7 KB | Supporting Information |
| anie202205159-sup-0001-misc_information.pdf6.3 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. J. Kochanowska-Karamyan, M. T. Hamann, Chem. Rev. 2010, 110, 4489–4497;
- 1bY. Wan, Y. Li, C. Yan, M. Yan, Z. Tang, Eur. J. Med. Chem. 2019, 183, 111691;
- 1cI. Cho, S. K. Park, B. Kang, J. W. Chung, J. H. Kim, K. Cho, S. Y. Park, Adv. Funct. Mater. 2016, 26, 2966–2973.
- 2
- 2aC. Zheng, S.-L. You, Acc. Chem. Res. 2020, 53, 974–987;
- 2bX. M. Xu, J. Z. Li, Z. L. Wang, Chin. J. Org. Chem. 2020, 40, 886–898;
- 2cJ. Qin, H. Zuo, Y. Ni, Q. Yu, F. Zhong, ACS Sustainable Chem. Eng. 2020, 8, 12342–12347;
- 2dM.-S. Tu, K.-W. Chen, P. Wu, Y.-C. Zhang, X.-Q. Liu, F. Shi, Org. Chem. Front. 2021, 8, 2643–2672.
- 3
- 3aR. Dalpozzo, Chem. Soc. Rev. 2015, 44, 742–778;
- 3bJ.-B. Chen, Y.-X. Jia, Org. Biomol. Chem. 2017, 15, 3550–3567;
- 3cY.-C. Zhang, F. Jiang, F. Shi, Acc. Chem. Res. 2020, 53, 425–446.
- 4For recent reviewers on catalytic atroposelective synthesis, see:
- 4aY.-B. Wang, B. Tan, Acc. Chem. Res. 2018, 51, 534–547;
- 4bJ. K. Cheng, S.-H. Xiang, S. Li, L. Ye, B. Tan, Chem. Rev. 2021, 121, 4805–4902;
- 4cJ. A. Carmona, C. Rodríguez-Franco, R. Fernández, V. Hornillos, J. M. Lassaletta, Chem. Soc. Rev. 2021, 50, 2968–2983.
- 5For recent studies on catalytic synthesis of indole-based atropisomers, see:
- 5aH.-H. Zhang, C.-S. Wang, C. Li, G.-J. Mei, Y. Li, F. Shi, Angew. Chem. Int. Ed. 2017, 56, 116–121; Angew. Chem. 2017, 129, 122–127;
- 5bL.-W. Qi, J.-H. Mao, J. Zhang, B. Tan, Nat. Chem. 2018, 10, 58–64;
- 5cM. Tian, D. Bai, G. Zheng, J. Chang, X. Li, J. Am. Chem. Soc. 2019, 141, 9527–9532;
- 5dF. Jiang, K.-W. Chen, P. Wu, Y.-C. Zhang, Y. Jiao, F. Shi, Angew. Chem. Int. Ed. 2019, 58, 15104–15110; Angew. Chem. 2019, 131, 15248–15254;
- 5eC. Ma, F. Jiang, F.-T. Sheng, Y. Jiao, G.-J. Mei, F. Shi, Angew. Chem. Int. Ed. 2019, 58, 3014–3020; Angew. Chem. 2019, 131, 3046–3052;
- 5fS. Zhu, Y.-H. Chen, Y.-B. Wang, P. Yu, S.-Y. Li, S.-H. Xiang, J.-Q. Wang, J. Xiao, B. Tan, Nat. Commun. 2019, 10, 4268;
- 5gL. Peng, K. Li, C. Xie, S. Li, D. Xu, W. Qin, H. Yan, Angew. Chem. Int. Ed. 2019, 58, 17199–17204; Angew. Chem. 2019, 131, 17359–17364;
- 5hX.-L. He, H.-R. Zhao, X. Song, B. Jiang, W. Du, Y.-C. Chen, ACS Catal. 2019, 9, 4374–4381;
- 5iW.-Y. Ding, P. Yu, Q.-J. An, K. L. Bay, S.-H. Xiang, S. Li, Y. Chen, K. N. Houk, B. Tan, Chem 2020, 6, 2046–2059;
- 5jY.-H. Chen, H.-H. Li, X. Zhang, S.-H. Xiang, S. Li, B. Tan, Angew. Chem. Int. Ed. 2020, 59, 11374–11378; Angew. Chem. 2020, 132, 11470–11474;
- 5kY.-P. He, H. Wu, Q. Wang, J. Zhu, Angew. Chem. Int. Ed. 2020, 59, 2105–2109; Angew. Chem. 2020, 132, 2121–2125;
- 5lA. Kim, A. Kim, S. Park, S. Kim, H. Jo, K. M. Ok, S. K. Lee, J. Song, Y. Kwon, Angew. Chem. Int. Ed. 2021, 60, 12279–12283; Angew. Chem. 2021, 133, 12387–12391;
- 5mL. Sun, H. Chen, B. Liu, J. Chang, L. Kong, F. Wang, Y. Lan, X. Li, Angew. Chem. Int. Ed. 2021, 60, 8391–8395; Angew. Chem. 2021, 133, 8472–8476.
- 6
- 6aW. Xia, Q.-J. An, S.-H. Xiang, S. Li, Y.-B. Wang, B. Tan, Angew. Chem. Int. Ed. 2020, 59, 6775–6779; Angew. Chem. 2020, 132, 6841–6845;
- 6bJ. Frey, A. Malekafzali, I. Delso, S. Choppin, F. Colobert, J. Wencel-Delord, Angew. Chem. Int. Ed. 2020, 59, 8844–8848; Angew. Chem. 2020, 132, 8929–8933;
- 6cQ. Ren, T. Cao, C. He, M. Yang, H. Liu, L. Wang, ACS Catal. 2021, 11, 6135–6140.
- 7
- 7aI. Takahashi, Y. Suzuki, O. Kitagawa, Org. Prep. Proced. Int. 2014, 46, 1–23;
- 7bZ. Li, S. Yu, Sci. Sin. Chim. 2020, 50, 509–525;
10.1360/SSC-2019-0168 Google Scholar
- 7cO. Kitagawa, Acc. Chem. Res. 2021, 54, 719–730;
- 7dP. Rodríguez-Salamanca, R. Fernández, V. Hornillos, J. M. Lassaletta, Chem. Eur. J. 2022, 28, e202104.
- 8
- 8aS. Brandes, M. Bella, A. Kjærsgaard, K. A. Jørgensen, Angew. Chem. Int. Ed. 2006, 45, 1147–1151; Angew. Chem. 2006, 118, 1165–1169;
- 8bH.-Y. Bai, F.-X. Tan, T.-Q. Liu, G.-D. Zhu, J.-M. Tian, T.-M. Ding, Z.-M. Chen, S.-Y. Zhang, Nat. Commun. 2019, 10, 3063;
- 8cD. Wang, Q. Jiang, X. Yang, Chem. Commun. 2020, 56, 6201–6204.
- 9G. X. Ortiz, Jr., B. N. Hemric, Q. Wang, Org. Lett. 2017, 19, 1314–1317.
- 10
- 10aM. M. Heravi, Z. Kheilkordi, V. Zadsirjan, M. Heydari, M. Malmir, J. Organomet. Chem. 2018, 861, 17–104;
- 10bR. Dorel, C. P. Grugel, A. M. Haydl, Angew. Chem. Int. Ed. 2019, 58, 17118–17129; Angew. Chem. 2019, 131, 17276–17287.
- 11
- 11aN. A. Romero, K. A. Margrey, N. E. Tay, D. A. Nicewicz, Science 2015, 349, 1326–1330;
- 11bK. A. Margrey, J. B. McManus, S. Bonazzi, F. Zecri, D. A. Nicewicz, J. Am. Chem. Soc. 2017, 139, 11288–11299;
- 11cN. Sauermann, R. Mei, L. Ackermann, Angew. Chem. Int. Ed. 2018, 57, 5090–5094; Angew. Chem. 2018, 130, 5184–5188;
- 11dQ.-L. Yang, X.-Y. Wang, J.-Y. Lu, L.-P. Zhang, P. Fang, T.-S. Mei, J. Am. Chem. Soc. 2018, 140, 11487–11494;
- 11eP. Feng, G. Ma, X. Chen, X. Wu, L. Lin, P. Liu, T. Chen, Angew. Chem. Int. Ed. 2019, 58, 8400–8404; Angew. Chem. 2019, 131, 8488–8492;
- 11fL. Zhang, L. Liardet, J. Luo, D. Ren, M. Gratzel, X. Hu, Nat. Catal. 2019, 2, 366–373;
- 11gA. Ruffoni, F. Julia, T. D. Svejstrup, A. J. McMillan, J. J. Douglas, D. Leonori, Nat. Chem. 2019, 11, 426–433.
- 12
- 12aS.-L. Li, C. Yang, Q. Wu, H.-L. Zheng, X. Li, J.-P. Cheng, J. Am. Chem. Soc. 2018, 140, 12836–12843;
- 12bH. Li, X. Yan, J. Zhang, W. Guo, J. Jiang, J. Wang, Angew. Chem. Int. Ed. 2019, 58, 6732–6736; Angew. Chem. 2019, 131, 6804–6808;
- 12cL. Wang, J. Zhong, X. Lin, Angew. Chem. Int. Ed. 2019, 58, 15824–15828; Angew. Chem. 2019, 131, 15971–15975;
- 12dS. D. Vaidya, S. T. Toenjes, N. Yamamoto, S. M. Maddox, J. L. Gustafson, J. Am. Chem. Soc. 2020, 142, 2198–2203;
- 12eT. Li, C. Mou, P. Qi, X. Peng, S. Jiang, G. Hao, W. Xue, S. Yang, L. Hao, Y. R. Chi, Angew. Chem. Int. Ed. 2021, 60, 9362–9367; Angew. Chem. 2021, 133, 9448–9453;
- 12fZ.-S. Liu, P.-P. Xie, Y. Hua, C. Wu, Y. Ma, J. Chen, H.-G. Cheng, X. Hong, Q. Zhou, Chem 2021, 7, 1917–1932;
- 12gR. Mi, H. Chen, X. Zhou, N. Li, D. Ji, F. Wang, Y. Lan, X. Li, Angew. Chem. Int. Ed. 2022, 61, e202111860; Angew. Chem. 2022, 134, e202111860.
- 13
- 13aV. Nair, R. S. Menon, A. T. Bijub, K. G. Abhilashc, Chem. Soc. Rev. 2012, 41, 1050–1059;
- 13bX. Zhang, Y.-H. Chen, B. Tan, Tetrahedron Lett. 2018, 59, 473–486.
- 14
- 14aL. Liao, C. Shu, M. Zhang, Y. Liao, X. Hu, Y. Zhang, Z. Wu, W. Yuan, X. Zhang, Angew. Chem. Int. Ed. 2014, 53, 10471–10475; Angew. Chem. 2014, 126, 10639–10643;
- 14bY.-H. Chen, D.-J. Cheng, J. Zhang, Y. Wang, X.-Y. Liu, B. Tan, J. Am. Chem. Soc. 2015, 137, 15062–15065;
- 14cJ.-Z. Wang, J. Zhou, C. Xu, H. Sun, L. Kürti, Q.-L. Xu, J. Am. Chem. Soc. 2016, 138, 5202–5205;
- 14dM. Moliterno, R. Cari, A. Puglisi, A. Antenucci, C. Sperio, E. Moretti, A. Di Sabato, R. Salvio, M. Bella, Angew. Chem. Int. Ed. 2016, 55, 6525–6529; Angew. Chem. 2016, 128, 6635–6639;
- 14eY.-H. Chen, L.-W. Qi, F. Fang, B. Tan, Angew. Chem. Int. Ed. 2017, 56, 16308–16312; Angew. Chem. 2017, 129, 16526–16530;
- 14fC. Xu, H. Zheng, B. Hu, X. Liu, L. Lin, X. Feng, Chem. Commun. 2017, 53, 9741–9744;
- 14gQ.-J. Liu, J. Zhu, X.-Y. Song, L. Wang, S. R. Wang, Y. Tang, Angew. Chem. Int. Ed. 2018, 57, 3810–3814; Angew. Chem. 2018, 130, 3872–3876;
- 14hS. N. Reddy, V. R. Reddy, S. Dinda, J. B. Nanubolu, R. Chra, Org. Lett. 2018, 20, 2572–2575;
- 14iW. Luo, Z. Sun, E. H. N. Ferno, V. N. Nesterov, T. R. Cundari, H. Wang, Chem. Sci. 2020, 11, 9386–9394;
- 14jG. Coombs, M. H. Sak, S. J. Miller, Angew. Chem. Int. Ed. 2020, 59, 2875–2880; Angew. Chem. 2020, 132, 2897–2902;
- 14kW.-Y. Ma, C. Gelis, D. Bouchet, P. Retailleau, X. Moreau, L. Neuville, G. Masson, Org. Lett. 2021, 23, 442–448.
- 15
- 15aE. Kumli, F. Montermini, P. Renaud, Org. Lett. 2006, 8, 5861–5864;
- 15bB. Xiong, R. Shen, M. Goto, S.-F. Yin, L.-B. Han, Chem. Eur. J. 2012, 18, 16902–16910;
- 15cX. Guo, H. Mayr, J. Am. Chem. Soc. 2014, 136, 11499–11512;
- 15dS. A. Konovalova, A. P. Avdeenko, A. A. Santalova, G. V. Palamarchuk, V. V. D'yakonenko, O. V. Shishkin, Russ. J. Org. Chem. 2015, 51, 42–50;
- 15eG. Song, Z. Zheng, Y. Wang, X. Yu, Org. Lett. 2016, 18, 6002–6005.
- 16
- 16aY. Huang, Y. Li, P.-H. Leung, T. Hayashi, J. Am. Chem. Soc. 2014, 136, 4865–4868;
- 16bG. A. Shevchenko, B. Oppelaar, B. List, Angew. Chem. Int. Ed. 2018, 57, 10756–10759; Angew. Chem. 2018, 130, 10916–10919.
- 17During the peer-review process of our manuscript, Liao and co-workers disclosed copper-catalyzed 1,6-addition of indoles and other heteroarenes to QDI in a racemate fashion, see: We. Lan, F. Liu, J. Hu, J. Zhu, S. Hu, J.-P. Wan, L. Liao, J. Org. Chem. 2022, 87, 5592–5602.
- 18Deposition Number 1974887 (for 3af) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 19R. Adams, A. S. Nagarkatti, J. Am. Chem. Soc. 1950, 72, 4601–4606.
- 20
- 20aQ. Yu, Y. Fu, J. Huang, J. Qin, H. Zuo, Y. Wu, F. Zhong, ACS Catal. 2019, 9, 7285–7291;
- 20bH. Zuo, J. Qin, W. Zhang, M. A. Bashir, Q. Yu, W. Zhao, G. Wu, F. Zhong, Org. Lett. 2020, 22, 6911–6916.
- 21
- 21aJ.-X. Zhang, F. K. Sheong, Z. Lin, Chem. Eur. J. 2018, 24, 9639–9650;
- 21bJ.-X. Zhang, F. K. Sheong, Z. Lin, WIREs Comput. Mol. Sci. 2020, 10, e1469.
- 22
- 22aW.-J. van Zeist, F. M. Bickelhaupt, Org. Biomol. Chem. 2010, 8, 3118–3127;
- 22bI. Fernández, F. M. Bickelhaupt, Chem. Soc. Rev. 2014, 43, 4953–4967.
- 23
- 23aK. D. Mane, A. Mukherjee, G. K. Das, G. Suryavanshi, J. Org. Chem. 2022, 87, 5097–5112.
- 24
- 24aM. Giambiagi, M. S. Giambiagi, K. C. Mundim, Struct. Chem. 1990, 1, 423;
- 24bM. Giambiagi, M. S. Giambiagi, C. D. S. Silva, A. P. Figueiredo, Phys. Chem. Chem. Phys. 2000, 2, 3381–3392.
- 25
- 25aJ. Klein, P. Fleurat-Lessard, J. Pilme, J. Comput. Chem. 2021, 42, 840–854;
- 25bH. Huang, L. Liu, J. Wang, Y. Zhou, H. Hu, X. Ye, G. Liu, Z. Xu, H. Xu, W. Yang, Y. Wang, Y. Peng, P. Yang, J. Sun, P. Yan, X. Cao, B. Tang, Chem. Sci. 2022, 13, 3129–3139.