Spatially Confined Intervention of Cellular Senescence by a Lysosomal Metabolism Targeting Molecular Prodrug for Broad-Spectrum Senotherapy
Yinghao Xia
Molecular Science and Biomedicine Laboratory (MBL), State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Aptamer Engineering Center of Hunan Province, Hunan University, Changsha, Hunan, 410082 China
These authors contributed equally to this work.
Search for more papers by this authorJili Li
Molecular Science and Biomedicine Laboratory (MBL), State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Aptamer Engineering Center of Hunan Province, Hunan University, Changsha, Hunan, 410082 China
These authors contributed equally to this work.
Search for more papers by this authorLinlin Wang
Molecular Science and Biomedicine Laboratory (MBL), State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Aptamer Engineering Center of Hunan Province, Hunan University, Changsha, Hunan, 410082 China
Search for more papers by this authorXiyuan Luo
Molecular Science and Biomedicine Laboratory (MBL), State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Aptamer Engineering Center of Hunan Province, Hunan University, Changsha, Hunan, 410082 China
Search for more papers by this authorYuqi Xie
Molecular Science and Biomedicine Laboratory (MBL), State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Aptamer Engineering Center of Hunan Province, Hunan University, Changsha, Hunan, 410082 China
Search for more papers by this authorCorresponding Author
Prof. Yanlan Liu
Molecular Science and Biomedicine Laboratory (MBL), State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Aptamer Engineering Center of Hunan Province, Hunan University, Changsha, Hunan, 410082 China
Search for more papers by this authorYinghao Xia
Molecular Science and Biomedicine Laboratory (MBL), State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Aptamer Engineering Center of Hunan Province, Hunan University, Changsha, Hunan, 410082 China
These authors contributed equally to this work.
Search for more papers by this authorJili Li
Molecular Science and Biomedicine Laboratory (MBL), State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Aptamer Engineering Center of Hunan Province, Hunan University, Changsha, Hunan, 410082 China
These authors contributed equally to this work.
Search for more papers by this authorLinlin Wang
Molecular Science and Biomedicine Laboratory (MBL), State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Aptamer Engineering Center of Hunan Province, Hunan University, Changsha, Hunan, 410082 China
Search for more papers by this authorXiyuan Luo
Molecular Science and Biomedicine Laboratory (MBL), State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Aptamer Engineering Center of Hunan Province, Hunan University, Changsha, Hunan, 410082 China
Search for more papers by this authorYuqi Xie
Molecular Science and Biomedicine Laboratory (MBL), State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Aptamer Engineering Center of Hunan Province, Hunan University, Changsha, Hunan, 410082 China
Search for more papers by this authorCorresponding Author
Prof. Yanlan Liu
Molecular Science and Biomedicine Laboratory (MBL), State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Aptamer Engineering Center of Hunan Province, Hunan University, Changsha, Hunan, 410082 China
Search for more papers by this authorGraphical Abstract
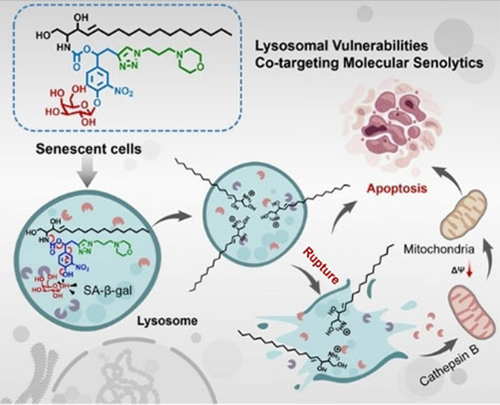
A spatially confined prodrug activation strategy is reported as a new paradigm of senotherapy. The prodrug is designed to target the defective lysosome metabolism, a unique yet ubiquitous event during cellular senescence, instead of dynamically changing and highly heterogeneous senescence-related signaling pathways employed in conventional methods, thereby fulfilling robust specificity and broad-spectrum activity towards senescent cells.
Abstract
Specific intervention of senescent cells (SnCs) is emerging as a powerful means to counteract aging and age-related diseases. Canonical methods are generally designed to target SnC-associated signaling pathways, which are however dynamically changing and highly heterogeneous in SnCs, significantly limiting the effectiveness. Here, we present a tailor-made molecular prodrug targeting lysosome dysfunction, a unique feature shared by virtually all types of SnCs. The prodrug comprises three modules all targeting the altered lysosomal programs in SnCs, namely, a recognizing unit towards the elevated lysosome content, a linker cleavable by the activated lysosomal enzyme, and a lysosomotropic agent targeting the increased lysosomal membrane sensitivity. This spatially confined design enables killing broad-spectrum SnCs, with high specificity over non-SnCs. Along with in vivo benefits, this work offers a way to significantly expand the applicability of senotherapy in a wide range of diseases.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202115764-sup-0001-misc_information.pdf10.9 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS. He, N. E. Sharpless, Cell 2017, 169, 1000–1011;
- 1bJ. M. van Deursen, Nature 2014, 509, 439–446.
- 2
- 2aJ. P. Coppé, P. Y. Desprez, A. Krtolica, J. Campisi, Annu. Rev. Pathol. 2010, 5, 99–118;
- 2bD. V. Faget, Q. Ren, S. A. Stewart, Nat. Rev. Cancer 2019, 19, 439–453.
- 3
- 3aB. Lozano-Torres, A. Estepa-Fernández, M. Rovira, M. Orzáez, M. Serrano, R. Martínez-Máñez, F. Sancenón, Nat. Chem. Rev. 2019, 3, 426–441;
- 3bD. J. Baker, T. Wijshake, T. Tchkonia, N. K. LeBrasseur, B. G. Childs, B. van de Sluis, J. L. Kirkland, J. M. van Deursen, Nature 2011, 479, 232–236.
- 4
- 4aY. Zhu, T. Tchkonia, T. Pirtskhalava, A. C. Gower, H. Ding, N. Giorgadze, A. K. Palmer, Y. Ikeno, G. B. Hubbard, M. Lenburg, S. P. O'Hara, N. F. LaRusso, J. D. Miller, C. M. Roos, G. C. Verzosa, N. K. LeBrasseur, J. D. Wren, J. N. Farr, S. Khosla, M. B. Stout, S. J. McGowan, H. Fuhrmann-Stroissnigg, A. U. Gurkar, J. Zhao, D. Colangelo, A. Dorronsoro, Y. Y. Ling, A. S. Barghouthy, D. C. Navarro, T. Sano, P. D. Robbins, L. J. Niedernhofer, J. L. Kirkland, Aging Cell 2015, 14, 644–658;
- 4bJ. Chang, Y. Wang, L. Shao, R. M. Laberge, M. Demaria, J. Campisi, K. Janakiraman, N. E. Sharpless, S. Ding, W. Feng, Y. Luo, X. Wang, N. Aykin-Burns, K. Krager, U. Ponnappan, M. Hauer-Jensen, A. Meng, D. Zhou, Nat. Med. 2016, 22, 78–83;
- 4cM. P. Baar, R. M. C. Brandt, D. A. Putavet, J. D. D. Klein, K. W. J. Derks, B. R. M. Bourgeois, S. Stryeck, Y. Rijksen, H. van Willigenburg, D. A. Feijtel, I. van der Pluijm, J. Essers, W. A. van Cappellen, W. F. van Willigenburg, A. B. Houtsmuller, J. Pothof, R. W. F. de Bruin, T. Madl, J. H. J. Hoeijmakers, J. Campisi, P. L. J. de Keizer, Cell 2017, 169, 132–147;
- 4dR. Yosef, N. Pilpel, R. Tokarsky-Amiel, A. Biran, Y. Ovadya, S. Cohen, E. Vadai, L. Dassa, E. Shahar, R. Condiotti, I. Ben-Porath, V. Krizhanovsky, Nat. Commun. 2016, 7, 11190.
- 5P. D. Robbins, D. Jurk, S. Khosla, J. L. Kirkland, N. K. LeBrasseur, J. D. Miller, J. F. Passos, R. J. Pignolo, T. Tchkonia, L. J. Niedernhofer, Annu. Rev. Pharmacol. Toxicol. 2021, 61, 779–803.
- 6
- 6aB. G. Childs, M. Gluscevic, D. J. Baker, R. M. Laberge, D. Marquess, J. Dananberg, J. M. van Deursen, Nat. Rev. Drug Discovery 2017, 16, 718–735;
- 6bA. Hernandez-Segura, T. V. de Jong, S. Melov, V. Guryev, J. Campisi, M. Demaria, Curr. Biol. 2017, 27, 2652–2660;
- 6cV. Gorgoulis, P. D. Adams, A. Alimonti, D. C. Bennett, O. Bischof, C. Bishop, J. Campisi, M. Collado, K. Evangelou, G. Ferbeyre, J. Gil, E. Hara, V. Krizhanovsky, D. Jurk, A. B. Maier, M. Narita, L. Niedernhofer, J. F. Passos, Cell 2019, 179, 813–827.
- 7
- 7aR. E. Lawrence, R. Zoncu, Nat. Cell Biol. 2019, 21, 133–142;
- 7bP. Saftig, J. Klumperman, Nat. Rev. Mol. Cell Biol. 2009, 10, 623–635;
- 7cC. Y. Lim, R. Zoncu, J. Cell Biol. 2016, 214, 653–664.
- 8A. Hernandez-Segura, J. Nehme, M. Demaria, Trends Cell Biol. 2018, 28, 436–453.
- 9
- 9aD. J. Kurz, S. Decary, Y. Hong, J. D. Erusalimsky, J. Cell Sci. 2000, 113, 3613–3622;
- 9bG. P. Dimri, X. Lee, G. Basile, M. Acosta, G. Scott, C. Roskelley, E. E. Medrano, M. Linskens, I. Rubelj, O. Pereira-Smith, Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367;
- 9cB. Y. Lee, J. A. Han, J. S. Im, A. Morrone, K. Johung, E. C. Goodwin, W. J. Kleijer, D. DiMaio, E. S. Hwang, Aging Cell 2006, 5, 187–195;
- 9dR. Gómez-Sintes, M. D. Ledesma, P. Boya, Ageing Res. Rev. 2016, 32, 150–168;
- 9eE. Robbins, E. M. Levine, H. Eagle, J. Exp. Med. 1970, 131, 1211–1222;
- 9fJ. T. Park, Y. S. Lee, K. A. Cho, S. C. Park, Ageing Res. Rev. 2018, 47, 176–182.
- 10
- 10aL. Groth-Pedersen, M. S. Ostenfeld, M. Hoyer-Hansen, J. Nylandsted, M. Jaattela, Cancer Res. 2007, 67, 2217–2225;
- 10bK. Ono, S. O. Kim, J. Han, Mol. Cell. Biol. 2003, 23, 665–676.
- 11K. Kagedal, M. Zhao, I. Svensson, U. T. Brunk, Biochem. J. 2001, 359, 335–343.
- 12
- 12aA. M. Villamil Giraldo, H. Appelqvist, T. Ederth, K. Ollinger, Biochem. Soc. Trans. 2014, 42, 1460–1464;
- 12bC. Ullio, J. Casas, U. T. Brunk, G. Sala, G. Fabrias, R. Ghidoni, G. Bonelli, F. M. Baccino, R. Autelli, J. Lipid Res. 2012, 53, 1134–1143.
- 13
- 13aE. González-Gualda, M. Pàez-Ribes, B. Lozano-Torres, D. Macias, J. R. Wilson 3rd, C. González-López, H. L. Ou, S. Mirón-Barroso, Z. Zhang, A. Lérida-Viso, J. F. Blandez, A. Bernardos, F. Sancenón, M. Rovira, L. Fruk, C. P. Martins, M. Serrano, G. J. Doherty, R. Martínez-Máñez, D. Muñoz-Espín, Aging Cell 2020, 19, e13142;
- 13bY. Cai, H. Zhou, Y. Zhu, Q. Sun, Y. Ji, A. Xue, Y. Wang, W. Chen, X. Yu, L. Wang, H. Chen, C. Li, T. Luo, H. Deng, Cell Res. 2020, 30, 574–589;
- 13cA. Guerrero, R. Guiho, N. Herranz, A. Uren, D. J. Withers, J. P. Martínez-Barbera, L. F. Tietze, J. Gil, Aging Cell 2020, 19, e13133.
- 14
- 14aM. Collado, M. A. Blasco, M. Serrano, Cell 2007, 130, 223–233;
- 14bJ. Campisi, Annu. Rev. Physiol. 2013, 75, 685–705.
- 15
- 15aM. Domenech, I. Marrero-Berrios, M. Torres-Lugo, C. Rinaldi, ACS Nano 2013, 7, 5091–5101;
- 15bY. Yang, M. Zhang, Y. Yang, D. Cheng, C. Yu, Angew. Chem. Int. Ed. 2021, 60, 11504–11513; Angew. Chem. 2021, 133, 11605–11614.
- 16
- 16aM. E. Guicciardi, M. Leist, G. J. Gores, Oncogene 2004, 23, 2881–2890;
- 16bP. Boya, G. Kroemer, Oncogene 2008, 27, 6434–6451.
- 17
- 17aG. Droga-Mazovec, L. Bojic, A. Petelin, S. Ivanova, R. Romih, U. Repnik, G. S. Salvesen, V. Stoka, V. Turk, B. Turk, J. Biol. Chem. 2008, 283, 19140–19150;
- 17bM. E. Guicciardi, J. Deussing, H. Miyoshi, S. F. Bronk, P. A. Svingen, C. Peters, S. H. Kaufmann, G. J. Gores, J. Clin. Invest. 2000, 106, 1127–1137;
- 17cM. Zhao, F. Antunes, J. W. Eaton, U. T. Brunk, Eur. J. Biochem. 2003, 270, 3778–3786.
- 18B. G. Childs, M. Durik, D. J. Baker, J. M. van Deursen, Nat. Med. 2015, 21, 1424–1435.