A Metal–Organic Framework as a Multiphoton Excitation Regulator for the Activation of Inert C(sp3)−H Bonds and Oxygen
Guanfeng Ji
State Key Laboratory of Fine Chemicals, Zhang Dayu School of Chemistry, Dalian University of Technology, Dalian, 116024 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Liang Zhao
State Key Laboratory of Fine Chemicals, Zhang Dayu School of Chemistry, Dalian University of Technology, Dalian, 116024 China
Search for more papers by this authorJianwei Wei
State Key Laboratory of Fine Chemicals, Zhang Dayu School of Chemistry, Dalian University of Technology, Dalian, 116024 China
Search for more papers by this authorDr. Junkai Cai
State Key Laboratory of Fine Chemicals, Zhang Dayu School of Chemistry, Dalian University of Technology, Dalian, 116024 China
Search for more papers by this authorProf. Dr. Cheng He
State Key Laboratory of Fine Chemicals, Zhang Dayu School of Chemistry, Dalian University of Technology, Dalian, 116024 China
Search for more papers by this authorZenggang Du
State Key Laboratory of Fine Chemicals, Zhang Dayu School of Chemistry, Dalian University of Technology, Dalian, 116024 China
Search for more papers by this authorWei Cai
State Key Laboratory of Fine Chemicals, Zhang Dayu School of Chemistry, Dalian University of Technology, Dalian, 116024 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Chunying Duan
State Key Laboratory of Fine Chemicals, Zhang Dayu School of Chemistry, Dalian University of Technology, Dalian, 116024 China
Search for more papers by this authorGuanfeng Ji
State Key Laboratory of Fine Chemicals, Zhang Dayu School of Chemistry, Dalian University of Technology, Dalian, 116024 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Liang Zhao
State Key Laboratory of Fine Chemicals, Zhang Dayu School of Chemistry, Dalian University of Technology, Dalian, 116024 China
Search for more papers by this authorJianwei Wei
State Key Laboratory of Fine Chemicals, Zhang Dayu School of Chemistry, Dalian University of Technology, Dalian, 116024 China
Search for more papers by this authorDr. Junkai Cai
State Key Laboratory of Fine Chemicals, Zhang Dayu School of Chemistry, Dalian University of Technology, Dalian, 116024 China
Search for more papers by this authorProf. Dr. Cheng He
State Key Laboratory of Fine Chemicals, Zhang Dayu School of Chemistry, Dalian University of Technology, Dalian, 116024 China
Search for more papers by this authorZenggang Du
State Key Laboratory of Fine Chemicals, Zhang Dayu School of Chemistry, Dalian University of Technology, Dalian, 116024 China
Search for more papers by this authorWei Cai
State Key Laboratory of Fine Chemicals, Zhang Dayu School of Chemistry, Dalian University of Technology, Dalian, 116024 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Chunying Duan
State Key Laboratory of Fine Chemicals, Zhang Dayu School of Chemistry, Dalian University of Technology, Dalian, 116024 China
Search for more papers by this authorGraphical Abstract
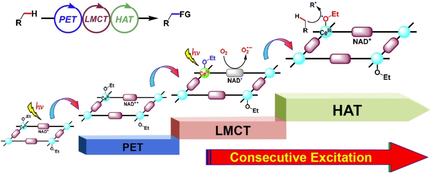
Through covalent modification of the nicotinamide adenine dinucleotide mimics and CeIII-OEt moieties in a metal–organic framework, a new multiphoton excitation approach was developed. This new strategy enables step-by-step triggering of the photoinduced electron transfer, ligand-to-metal charge transfer and hydrogen atom transfer processes for activation of inert C(sp3)−H bonds and oxygen.
Abstract
The activation and oxidization of inert C(sp3)−H bonds into value-added chemicals affords attractively economic and ecological benefits as well as central challenge in modern chemistry. Inspired by the natural enzymatic transformation, herein, we report a new multiphoton excitation approach to activate the inert C(sp3)−H bonds and oxygen by integrating the photoinduced electron transfer (PET), ligand-to-metal charge transfer (LMCT) and hydrogen atom transfer (HAT) events together into one metal-organic framework. The well-modified nicotinamide adenine dinucleotide (NAD+) mimics oxidized CeIII-OEt moieties to generate CeIV-OEt chromophore and its reduced state mimics NAD. via PET. The in situ formed CeIV-OEt moiety triggers a LMCT excitation to form the alkoxy radical EtO., abstracts a hydrogen atom from the C(sp3)−H bond, accompanying the recovery of CeIII-OEt and the formation of alkyl radicals. The formed NAD. activates oxygen to regenerate the NAD+ for next recycle, wherein, the activated oxygen species interacts with the intermediates for the oxidization functionalization, paving a catalytic avenue for developing scalable and sustainable synthetic strategy.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202114490-sup-0001-misc_information.pdf4.6 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aR. G. Bergman, Nature 2007, 446, 391–393;
- 1bN. J. Gunsalus, A. Koppaka, S. H. Park, S. M. Bischof, B. G. Hashiguchi, R. A. Periana, Chem. Rev. 2017, 117, 8521–8573;
- 1cD. J. Abrams, P. A. Provencher, E. J. Sorensen, Chem. Soc. Rev. 2018, 47, 8925–8967.
- 2
- 2aM.-Y. Qi, M. Conte, M. Anpo, Z.-R. Tang, Y.-J. Xu, Chem. Rev. 2021, 121, 13051—13085;
- 2bR. K. Zhang, K. Chen, X. Huang, L. Wohlschlager, H. Renata, F. H. Arnold, Nature 2019, 565, 67–72;
- 2cM. Borrome, S. Gronert, Angew. Chem. Int. Ed. 2019, 58, 14906–14910; Angew. Chem. 2019, 131, 15048–15052;
- 2dH. S. Sasmal, S. Bag, B. Chandra, P. Majumder, H. Kuiry, S. Karak, S. Sen Gupta, R. Banerjee, J. Am. Chem. Soc. 2021, 143, 8426–8436;
- 2eV. L. Sushkevich, D. Palagin, M. Ranocchiari, J. A. van Bokhoven, Science 2017, 356, 523–527.
- 3
- 3aY.-B. Huang, J. Liang, X.-S. Wang, R. Cao, Chem. Soc. Rev. 2017, 46, 126–157;
- 3bY. Liu, A. J. Howarth, N. A. Vermeulen, S.-Y. Moon, J. T. Hupp, O. K. Farha, Coord. Chem. Rev. 2017, 346, 101–111;
- 3cH. C. Zhou, J. R. Long, O. M. Yaghi, Chem. Rev. 2012, 112, 673–674.
- 4
- 4aJ. M. Mayer, Acc. Chem. Res. 2011, 44, 36–46;
- 4bC. Le, Y. Liang, R. W. Evans, X. Li, D. W. C. MacMillan, Nature 2017, 547, 79–83.
- 5
- 5aL. Capaldo, D. Ravelli, Eur. J. Org. Chem. 2017, 2056–2071;
- 5bX.-Z. Fan, J.-W. Rong, H.-L. Wu, Q. Zhou, H.-P. Deng, J. D. Tan, C.-W. Xue, L.-Z. Wu, H.-R. Tao, J. Wu, Angew. Chem. Int. Ed. 2018, 57, 8514–8518; Angew. Chem. 2018, 130, 8650–8654.
- 6
- 6aQ. Yang, Y.-H. Wang, Y. Qiao, M. Gau, P. J. Carroll, P. J. Walsh, E. J. Schelter, Science 2021, 372, 847–852;
- 6bA. Hu, J.-J. Guo, H. Pan, H. Tang, Z. Gao, Z. Zuo, J. Am. Chem. Soc. 2018, 140, 1612–1616;
- 6cB. J. Shields, A. G. Doyle, J. Am. Chem. Soc. 2016, 138, 12719–12722;
- 6dP. Xu, P. López-Rojas, T. Ritter, J. Am. Chem. Soc. 2021, 143, 5349–5354.
- 7S. Tsunoi, I. Ryu, N. Sonoda, J. Am. Chem. Soc. 1994, 116, 5473–5474.
- 8F. Glaser, C. Kerzig, O. S. Wenger, Angew. Chem. Int. Ed. 2020, 59, 10266–10284; Angew. Chem. 2020, 132, 10350–10370.
- 9
- 9aJ. Yuasa, S. Yamada, S. Fukuzumi, Angew. Chem. Int. Ed. 2008, 47, 1068–1071; Angew. Chem. 2008, 120, 1084–1087;
- 9bN. A. Romero, K. A. Margrey, N. E. Tay, D. A. Nicewicz, Science 2015, 349, 1326–1330.
- 10A. Hu, J.-J. Guo, H. Pan, Z. Zuo, Science 2018, 361, 668–672.
- 11Y. Qin, L. Zhu, S. Luo, Chem. Rev. 2017, 117, 9433–9520.
- 12L. Que, W. B. Tolman, Nature 2008, 455, 333–340.
- 13K. Keshari, M. Bera, L. Velasco, S. Munshi, G. Gupta, D. Moonshiram, S. Paria, Chem. Sci. 2021, 12, 4418–4424.
- 14Y. Quan, G. Lan, Y. Fan, W. Shi, E. You, W. Lin, J. Am. Chem. Soc. 2020, 142, 1746–1751.
- 15A. Spek, J. Appl. Crystallogr. 2003, 36, 7–13.
- 16X. Gong, Y. Shu, Z. Jiang, L. Lu, X. Xu, C. Wang, H. Deng, Angew. Chem. Int. Ed. 2020, 59, 5326–5331; Angew. Chem. 2020, 132, 5364–5369.
- 17X. Chen, H. Jiang, B. Hou, W. Gong, Y. Liu, Y. Cui, J. Am. Chem. Soc. 2017, 139, 13476–13482.
- 18H. Li, Y. Yang, C. He, L. Zeng, C. Duan, ACS Catal. 2019, 9, 422–430.
- 19S. M. Shin, M. S. Lee, J. H. Han, N. Jeong, Chem. Commun. 2014, 50, 289–291.
- 20Y. Shi, T. Zhang, X.-M. Jiang, G. Xu, C. He, C. Duan, Nat. Commun. 2020, 11, 5384–5393.
- 21L. M. Salonen, M. Ellermann, F. Diederich, Angew. Chem. Int. Ed. 2011, 50, 4808–4842; Angew. Chem. 2011, 123, 4908–4944.
- 22H. Yao, H. Ke, X. Zhang, S.-J. Pan, M.-S. Li, L.-P. Yang, G. Schreckenbach, W. Jiang, J. Am. Chem. Soc. 2018, 140, 13466–13477.
- 23
- 23aZ. Niu, W. Zhang, P. C. Lan, B. Aguila, S. Ma, Angew. Chem. Int. Ed. 2019, 58, 7420–7424; Angew. Chem. 2019, 131, 7498–7502;
- 23bZ. Jiang, X. Xu, Y. Ma, H. S. Cho, D. Ding, C. Wang, J. Wu, P. Oleynikov, M. Jia, J. Cheng, Y. Zhou, O. Terasaki, T. Peng, L. Zan, H. Deng, Nature 2020, 586, 549–554.
- 24Y. Qian, D. Li, Y. Han, H.-L. Jiang, J. Am. Chem. Soc. 2020, 142, 20763–20771.
- 25G. J. Kavarnos, Fundamentals of photoinduced electron transfer, Vol. 156, VCH, Weinheim, 1990, pp. 21–58.
- 26S. Smolders, A. Struyf, H. Reinsch, B. Bueken, T. Rhauderwiek, L. Mintrop, P. Kurz, N. Stock, D. E. De Vos, Chem. Commun. 2018, 54, 876–879.
- 27C. Zhang, Y. Xu, C. Lv, X. Zhou, Y. Wang, W. Xing, Q. Meng, Y. Kong, G. Chen, ACS Appl. Mater. Interfaces 2019, 11, 29917–29923.
- 28X. Wang, T. Saba, H. H. P. Yiu, R. F. Howe, J. A. Anderson, J. Shi, Chem 2017, 2, 621–654.
- 29A. Watanabe, D. B. McPhail, N. Maie, S. Kawasaki, H. A. Anderson, M. V. Cheshire, Org. Geochem. 2005, 36, 981–990.
- 30X. Sun, X. Luo, X. Zhang, J. Xie, S. Jin, H. Wang, X. Zheng, X. Wu, Y. Xie, J. Am. Chem. Soc. 2019, 141, 3797–3801.
- 31J. Zhang, S. Wu, X. Lu, P. Wu, J. Liu, ACS Nano 2019, 13, 14152–14161.
- 32H. Hu, Z. Wang, L. Cao, L. Zeng, C. Zhang, W. Lin, C. Wang, Nat. Chem. 2021, 13, 358–366.
- 33J. Jin, D. W. C. MacMillan, Angew. Chem. Int. Ed. 2015, 54, 1565–1569; Angew. Chem. 2015, 127, 1585–1589.
- 34J. Wang, Z. H. Zhu, M. W. Chen, Q. A. Chen, Y. G. Zhou, Angew. Chem. Int. Ed. 2019, 58, 1813–1817; Angew. Chem. 2019, 131, 1827–1831.
- 35J. A. Burkhard, G. Wuitschik, M. Rogers-Evans, K. Müller, E. M. Carreira, Angew. Chem. Int. Ed. 2010, 49, 9052–9067; Angew. Chem. 2010, 122, 9236–9251.
- 36
- 36aK. T. Smith, S. Berritt, M. González-Moreiras, S. Ahn, M. R. Smith, M.-H. Baik, D. J. Mindiola, Science 2016, 351, 1424–1427;
- 36bA. Caballero, E. Despagnet-Ayoub, M. Mar Díaz-Requejo, A. Díaz-Rodríguez, M. E. González-Núñez, R. Mello, B. K. Muñoz, W.-S. Ojo, G. Asensio, M. Etienne, P. J. Pérez, Science 2011, 332, 835–838.
- 37
- 37aI. Ghosh, T. Ghosh, J. I. Bardagi, B. König, Science 2014, 346, 725–728;
- 37bL. Zeng, T. Liu, C. He, D. Shi, F. Zhang, C. Duan, J. Am. Chem. Soc. 2016, 138, 3958–3961.
- 38T. Shen, T. H. Lambert, Science 2021, 371, 620–626.
- 39
- 39aE. M. Simmons, J. F. Hartwig, Angew. Chem. Int. Ed. 2012, 51, 3066–3072; Angew. Chem. 2012, 124, 3120–3126;
- 39bI. Garcia-Bosch, A. Company, C. W. Cady, S. Styring, W. R. Browne, X. Ribas, M. Costas, Angew. Chem. Int. Ed. 2011, 50, 5648–5653; Angew. Chem. 2011, 123, 5766–5771.
- 40A. Chatterjee, B. König, Angew. Chem. Int. Ed. 2019, 58, 14289–14294; Angew. Chem. 2019, 131, 14427–14432.
- 41W. Lee, S. Jung, M. Kim, S. Hong, J. Am. Chem. Soc. 2021, 143, 3003–3012.
- 42X. Li, A.-E. Surkus, J. Rabeah, M. Anwar, S. Dastigir, H. Junge, A. Brückner, M. Beller, Angew. Chem. Int. Ed. 2020, 59, 15849–15854; Angew. Chem. 2020, 132, 15983–15988.
- 43Y. Li, J. Wang, X.-M. Wang, F. Pan, T. Zhou, R.-J. Xie, J. Mater. Chem. C 2017, 5, 1022–1026.
- 44C. Tang, X. Qiu, Z. Cheng, N. Jiao, Chem. Soc. Rev. 2021, 50, 8067–8101.
- 45H. Fuse, H. Mitsunuma, M. Kanai, J. Am. Chem. Soc. 2020, 142, 4493–4499.
- 46Z. Guo, B. Liu, Q. Zhang, W. Deng, Y. Wang, Y. Yang, Chem. Soc. Rev. 2014, 43, 3480–3524.
- 47L. Ren, M.-M. Yang, C.-H. Tung, L.-Z. Wu, H. Cong, ACS Catal. 2017, 7, 8134–8138.
- 48J. Liu, X. Zhang, H. Yi, C. Liu, R. Liu, H. Zhang, K. Zhuo, A. Lei, Angew. Chem. Int. Ed. 2015, 54, 1261–1265; Angew. Chem. 2015, 127, 1277–1281.
- 49Deposition Numbers 2085233 (Ce-NAD+), 2085234 (Eu-NAD+), 2085235 (1@Ce-NAD+), 2085236 (2@Ce-NAD+), 2085237 (1&2@Ce-NAD+), and 2085238 (BA@Ce-NAD+) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.