Tailoring the Pore Surface of 3D Covalent Organic Frameworks via Post-Synthetic Click Chemistry
Dr. Bo Gui
Sauvage Center for Molecular Sciences, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, 430072 China
These authors contributed equally to this work.
Search for more papers by this authorXuefen Liu
Sauvage Center for Molecular Sciences, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, 430072 China
These authors contributed equally to this work.
Search for more papers by this authorYuanpeng Cheng
Sauvage Center for Molecular Sciences, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, 430072 China
Search for more papers by this authorYa Zhang
Sauvage Center for Molecular Sciences, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, 430072 China
Search for more papers by this authorPohua Chen
College of Chemistry and Molecular Engineering, Beijing National Laboratory for Molecular Sciences, Peking University, Beijing, 100871 China
Search for more papers by this authorMinghui He
Sauvage Center for Molecular Sciences, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, 430072 China
Search for more papers by this authorProf. Dr. Junliang Sun
College of Chemistry and Molecular Engineering, Beijing National Laboratory for Molecular Sciences, Peking University, Beijing, 100871 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Cheng Wang
Sauvage Center for Molecular Sciences, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, 430072 China
Search for more papers by this authorDr. Bo Gui
Sauvage Center for Molecular Sciences, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, 430072 China
These authors contributed equally to this work.
Search for more papers by this authorXuefen Liu
Sauvage Center for Molecular Sciences, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, 430072 China
These authors contributed equally to this work.
Search for more papers by this authorYuanpeng Cheng
Sauvage Center for Molecular Sciences, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, 430072 China
Search for more papers by this authorYa Zhang
Sauvage Center for Molecular Sciences, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, 430072 China
Search for more papers by this authorPohua Chen
College of Chemistry and Molecular Engineering, Beijing National Laboratory for Molecular Sciences, Peking University, Beijing, 100871 China
Search for more papers by this authorMinghui He
Sauvage Center for Molecular Sciences, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, 430072 China
Search for more papers by this authorProf. Dr. Junliang Sun
College of Chemistry and Molecular Engineering, Beijing National Laboratory for Molecular Sciences, Peking University, Beijing, 100871 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Cheng Wang
Sauvage Center for Molecular Sciences, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, 430072 China
Search for more papers by this authorDedicated to Professor Daoben Zhu and Professor J. Fraser Stoddart on the occasion of their 80th birthday
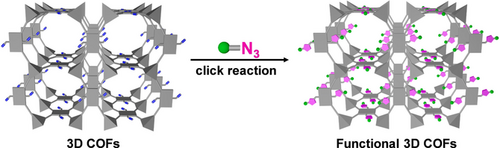
Graphical Abstract
A series of highly crystalline 3D COFs with variable loadings of ethynyl groups were designed and synthesized. Remarkably, these alkyne-tagged 3D COFs provide a platform for targeted anchoring various organic groups onto the pore walls via click reactions, which can accordingly change their properties, e.g., the obtained click products exhibited higher CO2/N2 selectivity.
Abstract
Three-dimensional covalent organic frameworks (3D COFs) have gained increasing attention for their attractive features. However, the development of 3D COFs is strongly restricted, mainly due to their synthetic difficulty and complicated structure determination. Post-synthetic modification, which can avoid these problems by incorporating functional moieties into a predetermined framework, provides an alternative way to construct 3D COFs with specific functions. Herein, we report the designed synthesis and characterization of a series of highly crystalline 3D COFs with different loadings of ethynyl groups. Notably, these alkyne-tagged 3D COFs provide a platform for targeted anchoring various specific groups onto the pore walls via click reactions. Moreover, the pore surface engineering can accordingly change their properties, for example, the obtained click products exhibited higher CO2/N2 selectivity. We describe a simple but powerful strategy to build functional 3D COFs, which will certainly advance them for a ranging of interesting applications in the future.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202113852-sup-0001-misc_information.pdf2.2 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS. Y. Ding, W. Wang, Chem. Soc. Rev. 2013, 42, 548–568;
- 1bC. S. Diercks, O. M. Yaghi, Science 2017, 355, eaal1585;
- 1cK. Geng, T. He, R. Liu, S. Dalapati, K. T. Tan, Z. Li, S. Tao, Y. Gong, Q. Jiang, D. Jiang, Chem. Rev. 2020, 120, 8814–8933;
- 1dB. Gui, G. Lin, H. Ding, C. Gao, A. Mal, C. Wang, Acc. Chem. Res. 2020, 53, 2225–2234.
- 2
- 2aY. Zeng, R. Zou, Y. Zhao, Adv. Mater. 2016, 28, 2855–2873;
- 2bL. Jiang, Y. Tian, T. Sun, Y. Zhu, H. Ren, X. Zou, Y. Ma, K. R. Meihaus, J. R. Long, G. Zhu, J. Am. Chem. Soc. 2018, 140, 15724–15730;
- 2cX. Guan, Y. Ma, H. Li, Y. Yusran, M. Xue, Q. Fang, Y. Yan, V. Valtchev, S. Qiu, J. Am. Chem. Soc. 2018, 140, 4494–4498;
- 2dH. Fan, M. Peng, I. Strauss, A. Mundstock, H. Meng, J. Caro, J. Am. Chem. Soc. 2020, 142, 6872–6877;
- 2eY. Ying, M. Tong, S. Ning, S. K. Ravi, S. B. Peh, S. C. Tan, S. J. Pennycook, D. Zhao, J. Am. Chem. Soc. 2020, 142, 4472–4480.
- 3
- 3aS.-Y. Ding, J. Gao, Q. Wang, Y. Zhang, W.-G. Song, C.-Y. Su, W. Wang, J. Am. Chem. Soc. 2011, 133, 19816–19822;
- 3bV. S. Vyas, F. Haase, L. Stegbauer, G. Savasci, F. Podjaski, C. Ochsenfeld, B. V. Lotsch, Nat. Commun. 2015, 6, 8508;
- 3cW. Liu, X. Li, C. Wang, H. Pan, W. Liu, K. Wang, Q. Zeng, R. Wang, J. Jiang, J. Am. Chem. Soc. 2019, 141, 17431–17440;
- 3dJ.-C. Wang, X. Kan, J.-Y. Shang, H. Qiao, Y.-B. Dong, J. Am. Chem. Soc. 2020, 142, 16915–16920.
- 4
- 4aG. Das, B. P. Biswal, S. Kandambeth, V. Venkatesh, G. Kaur, M. Addicoat, T. Heine, S. Verma, R. Banerjee, Chem. Sci. 2015, 6, 3931–3939;
- 4bM. R. Rao, Y. Fang, S. De Feyter, D. F. Perepichka, J. Am. Chem. Soc. 2017, 139, 2421–2427;
- 4cZ. Li, N. Huang, K. H. Lee, Y. Feng, S. Tao, Q. Jiang, Y. Nagao, S. Irle, D. Jiang, J. Am. Chem. Soc. 2018, 140, 12374–12377.
- 5
- 5aY. Du, H. Yang, J. M. Whiteley, S. Wan, Y. Jin, S.-H. Lee, W. Zhang, Angew. Chem. Int. Ed. 2016, 55, 1737–1741; Angew. Chem. 2016, 128, 1769–1773;
- 5bS. Wang, Q. Wang, P. Shao, Y. Han, X. Gao, L. Ma, S. Yuan, X. Ma, J. Zhou, X. Feng, B. Wang, J. Am. Chem. Soc. 2017, 139, 4258–4261;
- 5cC. Wu, Y. Liu, H. Liu, C. Duan, Q. Pan, J. Zhu, F. Hu, X. Ma, T. Jiu, Z. Li, Y. Zhao, J. Am. Chem. Soc. 2018, 140, 10016–10024;
- 5dX. Li, H. Wang, H. Chen, Q. Zheng, Q. Zhang, H. Mao, Y. Liu, S. Cai, B. Sun, C. Dun, M. P. Gordon, H. Zheng, J. A. Reimer, J. J. Urban, J. Ciston, T. Tan, E. M. Chan, J. Zhang, Y. Liu, Chem 2020, 6, 933–944.
- 6
- 6aX. Zhuang, W. Zhao, F. Zhang, Y. Cao, F. Liu, S. Bi, X. Feng, Polym. Chem. 2016, 7, 4176–4181;
- 6bS.-Y. Jiang, S.-X. Gan, X. Zhang, H. Li, Q.-Y. Qi, F.-Z. Cui, J. Lu, X. Zhao, J. Am. Chem. Soc. 2019, 141, 14981–14986;
- 6cY. Li, L. Guo, Y. Lv, Z. Zhao, Y. Ma, W. Chen, G. Xing, D. Jiang, L. Chen, Angew. Chem. Int. Ed. 2021, 60, 5363–5369; Angew. Chem. 2021, 133, 5423–5429;
- 6dQ. Sun, B. Aguila, P. C. Lan, S. Ma, Adv. Mater. 2019, 31, 1900008;
- 6eD. Bessinger, K. Muggli, M. Beetz, F. Auras, T. Bein, J. Am. Chem. Soc. 2021, 143, 7351–7357.
- 7H. M. El-Kaderi, J. R. Hunt, J. L. Mendoza-Cortés, A. P. Côté, R. E. Taylor, M. Keeffe, O. M. Yaghi, Science 2007, 316, 268–272.
- 8
- 8aG. Lin, H. Ding, D. Yuan, B. Wang, C. Wang, J. Am. Chem. Soc. 2016, 138, 3302–3305;
- 8bL. A. Baldwin, J. W. Crowe, D. A. Pyles, P. L. McGrier, J. Am. Chem. Soc. 2016, 138, 15134–15137;
- 8cO. Yahiaoui, A. N. Fitch, F. Hoffmann, M. Fröba, A. Thomas, J. Roeser, J. Am. Chem. Soc. 2018, 140, 5330–5333;
- 8dY. Chen, Z. L. Shi, L. Wei, B. Zhou, J. Tan, H. L. Zhou, Y. B. Zhang, J. Am. Chem. Soc. 2019, 141, 3298–3303;
- 8eY. Xie, J. Li, C. Lin, B. Gui, C. Ji, D. Yuan, J. Sun, C. Wang, J. Am. Chem. Soc. 2021, 143, 7279–7284.
- 9
- 9aT. Ma, E. A. Kapustin, S. X. Yin, L. Liang, Z. Zhou, J. Niu, L.-H. Li, Y. Wang, J. Su, J. Li, X. Wang, W. D. Wang, W. Wang, J. Sun, O. M. Yaghi, Science 2018, 361, 48–52;
- 9bC. Gao, J. Li, S. Yin, G. Lin, T. Ma, Y. Meng, J. Sun, C. Wang, Angew. Chem. Int. Ed. 2019, 58, 9770–9775; Angew. Chem. 2019, 131, 9872–9877.
- 10Y. Meng, Y. Luo, J.-L. Shi, H. Ding, X. Lang, W. Chen, A. Zheng, J. Sun, C. Wang, Angew. Chem. Int. Ed. 2020, 59, 3624–3629; Angew. Chem. 2020, 132, 3653–3658.
- 11Z. Ji, H. Wang, S. Canossa, S. Wuttke, O. M. Yaghi, Adv. Funct. Mater. 2020, 30, 2000238.
- 12
- 12aS. M. Cohen, Chem. Rev. 2012, 112, 970–1000;
- 12bJ. L. Segura, S. Royuela, M. Mar Ramos, Chem. Soc. Rev. 2019, 48, 3903–3945.
- 13
- 13aD. N. Bunck, W. R. Dichtel, Chem. Commun. 2013, 49, 2457–2459;
- 13bQ. Lu, Y. Ma, H. Li, X. Guan, Y. Yusran, M. Xue, Q. Fang, Y. Yan, S. Qiu, V. Valtchev, Angew. Chem. Int. Ed. 2018, 57, 6042–6048; Angew. Chem. 2018, 130, 6150–6156;
- 13cX. Han, J. Huang, C. Yuan, Y. Liu, Y. Cui, J. Am. Chem. Soc. 2018, 140, 892–895;
- 13dC. Gao, J. Li, S. Yin, J. Sun, C. Wang, Nat. Commun. 2020, 11, 4919.
- 14P. Wu, A. K. Feldman, A. K. Nugent, C. J. Hawker, A. Scheel, B. Voit, J. Pyun, J. M. J. Fréchet, K. B. Sharpless, V. V. Fokin, Angew. Chem. Int. Ed. 2004, 43, 3928–3932; Angew. Chem. 2004, 116, 4018–4022.
- 15C. Gao, J. Li, S. Yin, J. Sun, C. Wang, J. Am. Chem. Soc. 2020, 142, 3718–3723.
- 16
- 16aW. Wan, J. Sun, J. Su, S. Hovmöller, X. Zou, J. Appl. Crystallogr. 2013, 46, 1863–1873;
- 16bJ. Li, J. Sun, Acc. Chem. Res. 2017, 50, 2737–2745;
- 16cY. Wang, T. Yang, H. Xu, X. Zou, W. Wan, J. Appl. Crystallogr. 2018, 51, 1094–1101.
- 17R. L. Siegelman, E. J. Kim, J. R. Long, Nat. Mater. 2021, 20, 1060–1072.