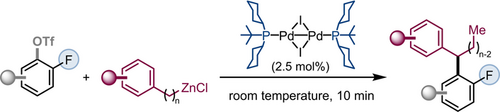
Air-Stable PdI Dimer Enabled Remote Functionalization: Access to Fluorinated 1,1-Diaryl Alkanes with Unprecedented Speed
Graphical Abstract
Abstract
While remote functionalization via chain walking has the potential to enable access to molecules via novel disconnections, such processes require relatively long reaction times and can be in need of elevated temperatures. This work features a remote arylation in less than 10 min reaction time at room temperature over a distance of up to 11 carbons. The unprecedented speed is enabled by the air-stable PdI dimer [Pd(μ-I)(PCy2tBu)]2, which in contrast to its PtBu3 counterpart does not trigger direct coupling at the initiation site, but regioconvergent and chemoselective remote functionalization to yield valuable fluorinated 1,1-diaryl alkanes. Our combined experimental and computational studies rationalize the origins of switchability, which are primarily due to differences in dispersion interactions.
The area of remote functionalization has seen a significant growth in recent years1 owing to its potential to enable structural access from diverse building blocks, especially in cases where the more established direct cross-coupling reactions are less amenable. This concept predominantly capitalizes on iterative β-hydride elimination/reinsertion steps,2 which translocate a metal catalyst along a carbon chain (via so-called “chain walking”) and can be initiated through, for example, hydrofunctionalization of a remote olefin or migratory cross-coupling (see Figure 1 A). While there has been impressive progress in recent years in this context,1, 3 the current methodological repertoire to access 1,1-diaryl alkanes via such a remote functionalization strategy4 requires relatively long reaction times (12–48 hours) and is limited in the substitution pattern; the vast majority of methods do not showcase the compatibility with ortho-substituents in these 1,1-diaryl alkanes.5 In this context Baudoin recently reported a notable advance which relied on Pd-catalysis and could tolerate ortho-substituents, albeit requiring high temperatures (120 °C) during 48 hours and a terminal blocking group in the substrate to prevent alternative products (see Figure 1 B).6, 7
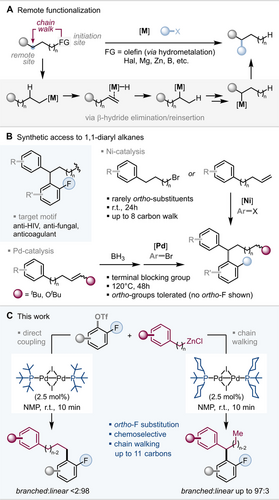
Remote functionalization, synthetic access to 1,1-diarylalkanes and this work.
Ortho-fluorinated 1,1-diaryl alkane motifs were not showcased. These motifs are of distinct importance, being featured in numerous bioactive compounds8 with anti-HIV, anti-fungal or anti-blood clotting activities, for example. More generally, the presence of fluorine often enhances metabolic stability, solubility as well as lipophilicity properties of organic molecules.9 However, the construction of ortho-fluorinated 1,1-diaryl alkanes via direct coupling approaches suffers either from the instability of ortho-fluorinated nucleophilic organometallic coupling partners, or in the alternative disconnection of benzylic substitution, from substrate dependence and functional group intolerance.10
The development of a remote functionalization approach to construct such ortho-fluorinated motifs in a rapid and modular fashion could therefore be greatly enabling.
We initially set out to test the reported chain walking methodology for the synthesis of 1,1-diaryl alkane substrates, which previously had reported selected examples with ortho-substituents5 to see if fluorine could potentially be tolerated. When we employed ortho-fluorinated aryl halides along with phenyl alkyl bromides or phenyl alkenes, we observed incomplete walks resulting in mixtures of isomers. Additionally, when there was steric hindrance present in the arene to which the walk occurred (e.g. o-Me or o-iPr arylalkyls), none of the desired 1,1-diaryl alkane product was obtained.11
With the goal to overcome these limitations and enable a modular access to ortho-fluorinated 1,1-diaryl alkanes, as well as push the boundaries of speed in the field of remote functionalization more generally, we set out to explore the reactivity of dinuclear PdI complexes in such migratory coupling reactions.
In our previous research, we could show that the air-stable and robust iodide-bridged PdI dimer [Pd(μ-I)(PtBu3)]2 enables the site-selective alkylation of poly(pseudo)halogenated arenes within minutes, using alkyl zinc reagents as nucleophilic coupling partners.12 The selectivity and speed were independent of the electronic and steric features exerted by the substrates, tolerating ortho-substituents as large as adamantyl,12c which contrasted typical Pd0-based strategies that generally lack this substrate-independence. In light of the generality and speed, we wondered whether these features could be transferred to the chain-walking concept, that is, in a migratory cross-coupling approach with a modified PdI dimer.
We recently reported that the air-stable PdI dimer [Pd(μ-I)(PCy2tBu)]2 enables E-selective olefin migration as well as C−C bond formations.13 Since remote functionalization often proceeds via migration of a double bond along a carbon chain, we wondered if this dimer [Pd(μ-I)(PCy2tBu)]2 could enable such a transformation (Figure 1 C).
To test our hypothesis, we added phenylpropyl-ZnCl along with PdI dimer [Pd(μ-I)(PCy2tBu)]2 to a solution of 3-chloro-2-fluorophenyl triflate in NMP. To our delight, we achieved regio- and chemoselective remote coupling at the OTf site within 10 minutes at room temperature to give 1 in excellent branched to linear selectivity (94:6) and 91 % isolated yield of the branched product (Figure 2).
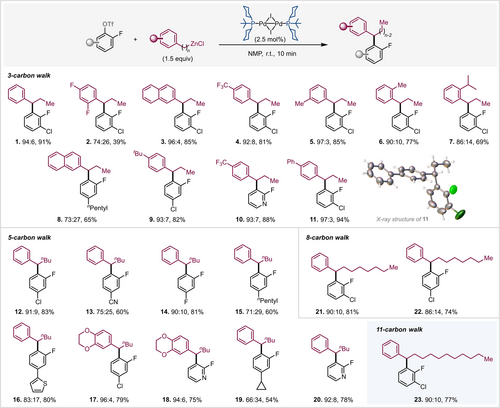
Scope of remote arylation. Reaction conditions: aryl triflate (0.2 mmol), [Pd(μ-I)(PCy2tBu)]2 (0.005 mmol, 2.5 mol %), organozinc reagent (0.24 mmol, 1.2 equiv, in THF) in NMP (1.5 mL) for 10 min at r.t. under argon. Ratios of branched to linear product and isolated yields of branched products are given.11, 14
To the best of our knowledge, this reaction speed paired with selectivity is completely unprecedented. By contrast, using the PtBu3-derived dimer instead, that is, [Pd(μ-I)(PtBu3)]2, we obtained direct coupling to the linear product 26 in the same speed, without any migration, in line with our previous research (Figure 3 A).12e As such, a subtle ligand modification within the PdI dimer framework can fully switch the reaction outcome towards the branched product, while retaining the beneficial features of air-stability and reaction speed.
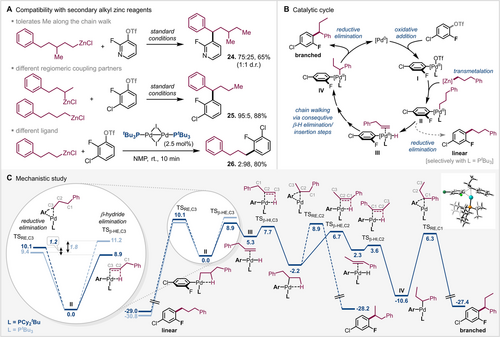
a) Compatibility of the method with secondary alkyl zinc reagents; b) Catalytic cycle; c) Mechanistic study. Energies (in kcal mol−1) refer to Gibbs free energies relative to PdII obtained after transmetalation, calculated at the SMD (DMAc) M06-2X-D3/def2-TZVP//MN15-D3(BJ)/def2-SVP level of theory.11, 15
We next set out to explore the generality of this transformation (Figure 2). In this context, we primarily focused on ortho-fluoro aryl triflate substrates that contained also C-Cl sites to create additional possibilities for downstream diversification. In all cases coupling was exclusively observed at the C-OTf site; the competing C-Cl site remained untouched.16
Variation of the alkyl zinc reagents in terms of chain length had no impact on the transformation; irrespective of chain length we achieved good yields and high regioselectivities within 10 minutes at room temperature. Electronic variations of the aryl group of the coupling partner are well tolerated, ranging from electron donating tert-butyl (9) to electron withdrawing trifluoromethyl-substituted aryl moieties (4, 10). Notably, the ortho-methyl and -isopropyl groups (6, 7) did not affect the reaction speed and efficiency, and only minimally effected the product selectivity (branched vs. linear). While as large substituents as isopropyl have so far not been showcased, the smaller ortho-methyl substituent (as in 6) could otherwise only be tolerated under much harsher conditions, such as Baudoin's recent protocol,6 requiring 120 °C and 48 h reaction time or Zhu's protocol,5c needing 80 °C for 24 h. Potentially coordinating functionalities such as the cyano group (13) did not impede the reaction. Heterocycles (10, 18 and 20) were also equally effective.
Notably, the presence of a methyl group along the chain did not markedly impede the walk (24). Although a slightly lower branched to linear ratio was obtained, the branched product could be isolated in a good yield of 65 % as a 1:1 mixture of diastereomers (Figure 3 A).
Finally, to test the applicability of our method for secondary rather than primary alkyl zinc reagents, we employed an equal mixture of primary and secondary alkyl zinc bromide as a starting material (Figure 3 A). The reaction proceeded with high selectivity (95:5) and gave the branched 1,1-diaryl product 25 in high yield (88 %).
Intrigued by the unprecedented speed and high regioselectivity, we subsequently turned to computational studies to gain insight. We previously reviewed that depending on the precise reaction conditions as well as bridging halide of the PdI dimer, different active catalytic species can result, ranging from direct reactivity of the PdI dimer to being a precursor for Pd0 or PdII-H species.17 Our previous studies concluded that under the herein employed conditions (i.e. organometallic coupling partner in NMP), the reactivity (and selectivity) for the C(sp2)-OTf oxidative addition is consistent with a Pd0 species, either an anionic Pd ate complex or solvent coordinated (sol)Pd0L.12e, 18 Following oxidative addition, a monophosphine PdII intermediate I will result (see Figure 3 B). After transmetalation, reductive elimination to the linear product can potentially occur from the resulting intermediate II or β-H elimination can initiate the chain walk. Our calculations19 at the SMD (DMAc) M06-2X-D3/def2-TZVP//MN15-D3(BJ)/def2-SVP level of theory15 of the coupling of ortho-fluoro-para-chlorophenyl triflate with phenylpropyl-ZnCl indicate that while the reductive elimination towards the linear product is clearly favored for the PtBu3 ligand (by ΔΔG≠=1.8 kcal mol−1), in the case of the PCy2tBu ligand, the competing β-hydride elimination is favored (by ΔΔG≠=1.2 kcal mol−1), which is consistent with the observed product divergence. The subsequent chain walk via consecutive β-hydride elimination and reinsertion steps (see Figure 3 C) is also of lower activation free energy barrier than the reductive elimination towards the linear product.11 The lowest transition state for an irreversible reductive elimination from a PdII-alkyl intermediate along the walk likely determines the product selectivity. Comparing the three possible reductive elimination transition states showed that indeed formation of branched 1,1-diaryl is favored over the linear analogues by ΔΔG≠=2.6 (TSRE,C1 vs. TSRE,C2) and 3.8 (TSRE,C1 vs. TSRE,C3) kcal mol−1, respectively.11
As such, the calculations are fully consistent with the observed product selectivities. To gain greater insight on the origins of the subtle ligand impacts, we performed a distortion/interaction analysis20 of the reductive elimination and β-hydride elimination transition states (see supporting information for details). Our analysis indicated that while there was no marked difference in the fragment energies for the two ligands in the reductive elimination, there is a difference in interaction energies in the β-H elimination transition state. For PCy2tBu we determined a 4.2 kcal mol−1 greater interaction energy compared to PtBu3. Our further analysis of the underlying non-covalent interactions (NCI analysis) indicated that the primary origin of this increased interaction energy is dispersion. With other words, the chain walking is favored for PCy2tBu as a consequence of enhanced stabilizing dispersion forces.
Aside from the product selectivity, the overall reaction speed of only 10 min at room temperature is unprecedented also (as compared to 24 h reaction times in Ni-based couplings or 48 h in Pd-based protocols).4, 5, 6 Since the chain walking relies on the analogous elementary steps as other Pd-based protocols (i.e. PdII β-H elimination and reinsertion, see Figure 3 A), the unprecedented overall speed implies that the pre-catalyst and its speciation in solution, does not only influence the release of the active catalytic Pd0 species, but also its fate and availability in later catalytic turnover. The dynamic interplay of the various components (speciation) in solution clearly effects the entire transformation, which is an observation that has not been the focus in homogenous catalysis so far. With the PdI dimer entity being rapidly and nucleophilically activated17, 21 to the monophosphine Pd0 species without dynamic association/dissociation events of competing phosphine ligands (which frequently is the case in Pd0 based protocols that rely on initial ligand dissociation steps), the individual elementary steps as well as later catalyst turnover also appear to be unaffected by competing events in the PdI dimer protocol. These results suggest that the nature of the pre-catalyst and overall reservoir of active species should therefore shift into greater focus and be considered more closely in the future development of remote functionalization strategies.
In conclusion, we have demonstrated a chemo- and regioselective remote functionalization strategy for the modular access to ortho-fluorinated 1,1-diaryl alkanes. The process is enabled by an air-stable PdI dimer and couples alkyl organozinc reagents with aryl triflates that possess ortho-fluoro substituents as well as competing C-Cl sites. The migratory cross-coupling proceeds with unprecedented speed in only 10 min at room temperature for up to 11 carbon chains and is tolerant of various functionalities and substitution patterns that are not accessible with alternative chain-walking protocols. Our computational investigation indicates that facile β-hydride elimination and reinsertion steps allow for a rapid chain walking, which is primarily a consequence of greater dispersive interaction with PCy2tBu, and the low barrier for the reductive elimination step is the origin of selectivity for the formation of benzylic branched product. More generally, the crucial role of the reservoir of the active catalytic species on the overall reaction speed and efficiency is showcased, an aspect that has so far not been a focus in the area of remote functionalization.
Acknowledgements
We thank the European Research Council (ERC-637993) for funding. Calculations were performed with computing resources granted by JARA-HPC from RWTH Aachen University under project “jara0091”. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.