Dynamic Optical Visualization of Proton Transport Pathways at Water–Solid Interfaces
Dr. Jinmei Yang
Laboratory of Experimental Physical Biology, Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Yuxian Lu
Laboratory of Experimental Physical Biology, Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Lei Jin
College of Pharmaceutical Sciences, Zhejiang University, Hangzhou, 310058 China
Search for more papers by this authorChunxiao Zhao
Laboratory of Experimental Physical Biology, Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorYuang Chen
Laboratory of Experimental Physical Biology, Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorYang Xu
Laboratory of Experimental Physical Biology, Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorFanfan Chen
Laboratory of Experimental Physical Biology, Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorCorresponding Author
Prof. Jiandong Feng
Laboratory of Experimental Physical Biology, Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorDr. Jinmei Yang
Laboratory of Experimental Physical Biology, Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Yuxian Lu
Laboratory of Experimental Physical Biology, Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Lei Jin
College of Pharmaceutical Sciences, Zhejiang University, Hangzhou, 310058 China
Search for more papers by this authorChunxiao Zhao
Laboratory of Experimental Physical Biology, Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorYuang Chen
Laboratory of Experimental Physical Biology, Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorYang Xu
Laboratory of Experimental Physical Biology, Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorFanfan Chen
Laboratory of Experimental Physical Biology, Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorCorresponding Author
Prof. Jiandong Feng
Laboratory of Experimental Physical Biology, Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorGraphical Abstract
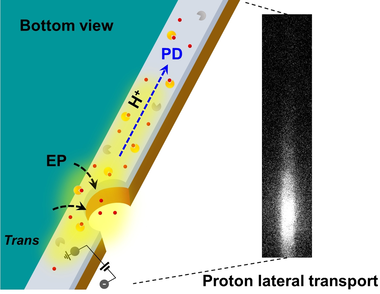
We developed a method for imaging aqueous proton transport with spatial resolution and observed the lateral transport of protons along the nanofluidic interface. The dynamics of proton transport can be further captured by quantitative analysis, thanks to the temporal resolution of this optical imaging technique.
Abstract
Probing proton transport is of vital importance for understanding cellular transport, surface catalysis and fuel cells. Conventional proton transport measurements rely on the use of electrochemical conductivity and do not allow for the direct visualization of proton transport pathways. The development of novel experimental techniques to spatiotemporally resolve proton transport is in high demand. Here, building upon the general conversion of aqueous proton flux into spatially resolved fluorescence signals, we optically visualize proton transport through nanopores and along hydrophilic interfaces. We observed that the fluorescence intensity increased at negative voltage due to lateral transport. Thanks to the temporal resolution of optical imaging, our technique further empowers the analysis of proton transport dynamics.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202112150-sup-0001-misc_information.pdf10.1 MB | Supporting Information |
| anie202112150-sup-0001-Video_1.mov94 KB | Supporting Information |
| anie202112150-sup-0001-Video_2.mov465.6 KB | Supporting Information |
| anie202112150-sup-0001-Video_3.mov584.1 KB | Supporting Information |
| anie202112150-sup-0001-Video_4.mov58.7 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C. J. T. Grotthuss, Ann. Chim. 1806, LVIII, 54–74.
- 2D. Marx, ChemPhysChem 2006, 7, 1848–1870.
- 3N. Agmon, Chem. Phys. Lett. 1995, 244, 456–462.
- 4J. Heberle, N. A. Dencher in Proton Transfer in Hydrogen-Bonded Systems. NATO ASI Series (Series B: Physics), Vol. 291, (Ed.: T. Bountis), Springer, Boston, MA, 1992.
- 5A. Badalyan, S. S. Stahl, Nature 2016, 535, 406–410.
- 6K. D. Kreuer, S. J. Paddison, E. Spohr, M. Schuster, Chem. Rev. 2004, 104, 4637–4678.
- 7M. Brändén, T. Sandén, P. Brzezinski, J. Widengren, Proc. Natl. Acad. Sci. USA 2006, 103, 19766–19770.
- 8R. H. Tunuguntla, F. I. Allen, K. Kim, A. Belliveau, A. Noy, Nat. Nanotechnol. 2016, 11, 639–644.
- 9S. Hu, M. Lozada-Hidalgo, F. C. Wang, A. Mischenko, F. Schedin, R. R. Nair, E. W. Hill, D. V. Boukhvalov, M. I. Katsnelson, R. A. W. Dryfe, I. V. Grigorieva, H. A. Wu, A. K. Geim, Nature 2014, 516, 227–230.
- 10L. Mogg, G.-P. Hao, S. Zhang, C. Bacaksiz, Y. Zou, S. J. Haigh, F. M. Peeters, A. K. Geim, M. Lozada-Hidalgo, Nat. Nanotechnol. 2019, 14, 962–966.
- 11V. A. Magnottaa, H.-Y. Heo, B. J. Dlouhy, N. S. Dahdaleh, R. L. Follmer, D. R. Thedens, M. J. Welsh, J. A. Wemmie, Proc. Natl. Acad. Sci. USA 2012, 109, 8270–8273.
- 12L. R. Merte, G. Peng, R. Bechstein, F. Rieboldt, C. A. Farberow, L. C. Grabow, W. Kudernatsch, S. Wendt, E. Lægsgaard, M. Mavrikakis, F. Besenbacher, Science 2012, 336, 889–893.
- 13S. Veshaguri, S. M. Christensen, G. C. Kemmer, G. Ghale, M. P. Møller, C. Lohr, A. L. Christensen, B. H. Justesen, I. L. Jørgensen, J. Schiller, N. S. Hatzakis, M. Grabe, T. G. Pomorski, D. Stamou, Science 2016, 351, 1469–1473.
- 14C. Zhang, D. G. Knyazev, Y. A. Vereshaga, E. Ippoliti, T. H. Nguyen, P. Carloni, P. Pohl, Proc. Natl. Acad. Sci. USA 2012, 109, 9744–9749.
- 15J. Comtet, B. Grosjean, E. Glushkov, A. Avsar, K. Watanabe, T. Taniguchi, R. Vuilleumier, M.-L. Bocquet, A. Radenovic, Nat. Nanotechnol. 2020, 15, 598–604.
- 16L. Xue, H. Yamazaki, R. Ren, M. Wanunu, A. P. Ivanov, J. B. Edel, Nat. Rev. Mater. 2020, 5, 931–951.
- 17Z. Diwu, C.-S. Chen, C. Zhang, D. H. Klaubert, R. P. Haugland, Chem. Biol. 1999, 6, 411–418.
- 18H. M. DePedro, P. Urayama, Anal. Biochem. 2009, 384, 359–361.
- 19M. Wanunu, A. Meller, Nano Lett. 2007, 7, 1580–1585.
- 20B. N. Anderson, O. N. Assad, T. Gilboa, A. H. Squires, D. Bar, A. Meller, ACS Nano 2014, 8, 11836–11845.
- 21A. Ivankin, R. Y. Henley, J. Larkin, S. Carson, M. L. Toscano, M. Wanunu, ACS Nano 2014, 8, 10774–10781.
- 22S. Huang, M. Romero-Ruiz, O. K. Castell, H. Bayley, M. I. Wallace, Nat. Nanotechnol. 2015, 10, 986–991.
- 23J. Y. Jung, P. Joshi, L. Petrossian, T. J. Thornton, J. D. Posner, Anal. Chem. 2009, 81, 3128–3133.
- 24J. F. Perez, M. C. Rui, F. Michelangeli, J. Physiol. 2001, 537, 735–745.
- 25Y. Yamada, T. Ikuta, T. Nishiyama, K. Takahashi, Y. Takata, Langmuir 2014, 30, 14532–14537.
- 26S. Serowy, S. M. Saparov, Y. N. Antonenko, W. Kozlovsky, V. Hagen, P. Pohl, Biophys. J. 2003, 84, 1031–1037.
- 27A. Springer, V. Hagen, D. A. Cherepanov, Y. N. Antonenko, P. Pohl, Proc. Natl. Acad. Sci. USA 2011, 108, 14461–14466.
- 28Y. N. Antonenko, P. Pohl, Eur. Biophys. J. 2008, 37, 865–870.
- 29E. S. Medvedev, A. A. Stuchebrukhov, FEBS Lett. 2013, 587, 345–349.
- 30E. S. Medvedev, A. A. Stuchebrukhov, J. Math. Biol. 2006, 52, 209–234.
- 31E. Weichselbaum, M. Österbauer, D. G. Knyazev, O. V. Batishchev, S. A. Akimov, T. H. Nguyen, C. Zhang, G. Knör, N. Agmon, P. Carloni, P. Pohl, Sci. Rep. 2017, 7, 4553.
- 32J. L. Achtyl, R. R. Unocic, L. Xu, Y. Cai, M. Raju, W. Zhang, R. L. Sacci, I. V. Vlassiouk, P. F. Fulvio, P. Ganesh, D. J. Wesolowski, S. Dai, A. C. T. van Duin, M. Neurock, F. M. Geiger, Nat. Commun. 2015, 6, 6539.
- 33A. Bagusetty, P. Choudhury, W. A. Saidi, B. Derksen, E. Gatto, J. K. Johnson, Phys. Rev. Lett. 2017, 118, 186101.
- 34J. Carrasco, A. Hodgson, A. Michaelides, Nat. Mater. 2012, 11, 667–674.
- 35Y. Jing, N. Aluru, J. Power Sources 2020, 445, 227327.