Palladium/Xiao-Phos-Catalyzed Kinetic Resolution of sec-Phosphine Oxides by P-Benzylation
Qiang Dai
School of Chemistry and Molecular Engineering and Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, East China Normal University, Shanghai, 200241 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Lu Liu
School of Chemistry and Molecular Engineering and Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, East China Normal University, Shanghai, 200241 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Junliang Zhang
Department of Chemistry, Fudan University, 2005 Songhu Road, Shanghai, 200438 P. R. China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 P. R. China
Search for more papers by this authorQiang Dai
School of Chemistry and Molecular Engineering and Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, East China Normal University, Shanghai, 200241 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Lu Liu
School of Chemistry and Molecular Engineering and Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, East China Normal University, Shanghai, 200241 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Junliang Zhang
Department of Chemistry, Fudan University, 2005 Songhu Road, Shanghai, 200438 P. R. China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 P. R. China
Search for more papers by this authorDedicated to 70th anniversary of East China Normal University
Graphical Abstract
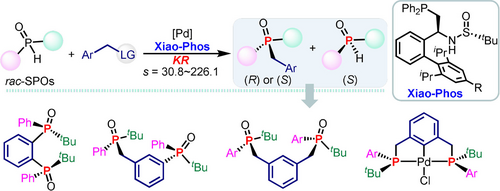
A kinetic resolution of rac-secondary phosphine oxides via the enantioselective P-benzylation process catalyzed by the palladium/Xiao-Phos was designed. Both, tert- and sec-phosphine oxides were delivered in good yield and excellent enantiopurity (selectivity factor up to 226.1). The synthetic utilities are further demonstrated by the facile preparation of several P-chiral compounds, precursors of bidentate ligands, and transition metal complexes.
Abstract
P-stereogenic tert- and sec-phosphines have wide applications in asymmetric catalysis, materials, and pharmaceutical chemistry, however, their practical synthesis still constitutes a significant challenge. Herein, a successful kinetic resolution of rac-secondary phosphine oxides via the enantioselective P-benzylation process catalyzed by the palladium/Xiao-Phos was designed. Both tert- and sec-phosphine oxides were delivered in good yield and excellent enantiopurity (selectivity factor up to 226.1). The appealing synthetic utilities are further demonstrated by the facile preparation of several valuable P-chiral compounds, precursors of bidentate ligands, as well as transition metal complexes.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202111957-sup-0001-cif.zip1.8 MB | Supporting Information |
| anie202111957-sup-0001-misc_information.pdf16.4 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. Börner, Phosphorus Ligands in Asymmetric Catalysis; Synthesis and Applications, Wiley-VCH, Weinheim, 2008, pp. 1–10;
- 1bM. Dutartre, J. Bayardon, S. Juge, Chem. Soc. Rev. 2016, 45, 5771–5794;
- 1cT. Imamoto, Chem. Rec. 2016, 16, 2659–2673;
- 1dW.-M. Dai, K. K. Y. Yeung, W. H. Leung, R. K. Haynes, Tetrahedron: Asymmetry 2003, 14, 2821–2826;
- 1eJ. L. Methot, W. R. Roush, Adv. Synth. Catal. 2004, 346, 1035–1050;
- 1fJ. Seayad, B. List, Org. Biomol. Chem. 2005, 3, 719–724;
- 1gS. J. Connon, Angew. Chem. Int. Ed. 2006, 45, 3909–3912; Angew. Chem. 2006, 118, 4013–4016;
- 1hE. Remond, S. Juge, Chem. Today 2014, 32, 49–55;
- 1iH. Guo, Y. C. Fan, Z. Sun, Y. Wu, O. Kwon, Chem. Rev. 2018, 118, 10049–10293;
- 1jH. Ni, W.-L. Chan, Y. Lu, Chem. Rev. 2018, 118, 9344–9411.
- 2
- 2aS. J. Hecker, M. D. Erion, J. Med. Chem. 2008, 51, 2328–2345;
- 2bL. Clarion, C. Jacquard, O. Sainte-Catherine, M. Decoux, S. Loiseau, M. Rolland, M. Lecouvey, J.-P. Hugnot, J.-N. Volle, D. Virieux, J.-L. Pirat, N. Bakalara, J. Med. Chem. 2014, 57, 8293–8306;
- 2cU. Pradere, E. C. Garnier-Amblard, S. J. Coats, F. Amblard, R. F. Schinazi, Chem. Rev. 2014, 114, 9154–9218;
- 2dN. Iwamoto, D. C. D. Butler, N. Svrzikapa, S. Mohapatra, I. Zlatev, D. W. Y. Sah, Meena, S. M. Standley, G. Lu, L. H. Apponi, M. Frank-Kamenetsky, J. J. Zhang, C. Vargeese, G. L. Verdine, Nat. Biotechnol. 2017, 35, 845–851;
- 2eY. Mehellou, H. S. Rattan, J. Balzarini, J. Med. Chem. 2018, 61, 2211–2226.
- 3For reviews, see:
- 3aK. M. Pietrusiewicz, M. Zablocka, Chem. Rev. 1994, 94, 1375–1411;
- 3bA. Grabulosa, J. Granell, G. Muller, Coord. Chem. Rev. 2007, 251, 25–90;
- 3cO. I. Kolodiazhnyi, Tetrahedron: Asymmetry 2012, 23, 1–46; For selected examples, see:
- 3dE. Bergin, C. T. O'Connor, S. B. Robinson, E. M. McGarrigle, C. P. O'Mahony, D. G. Gilheany, J. Am. Chem. Soc. 2007, 129, 9566–9567;
- 3eZ. S. Han, N. Goyal, M. A. Herbage, J. D. Sieber, B. Qu, Y. Xu, Z. Li, J. T. Reeves, J.-N. Desrosiers, S. Ma, N. Grinberg, H. Lee, H. P. R. Mangunuru, Y. Zhang, D. Krishnamurthy, B. Z. Lu, J. J. Song, G. Wang, C. H. Senanayake, J. Am. Chem. Soc. 2013, 135, 2474–2477;
- 3fP. Du, X.-B. Lu, Eur. J. Org. Chem. 2020, 5003–5008;
- 3gZ. You, K. Higashida, T. Iwai, M. Sawamura, Angew. Chem. Int. Ed. 2021, 60, 5778–5782; Angew. Chem. 2021, 133, 5842–5846.
- 4For reviews, see:
- 4aD. S. Glueck, Synlett 2007, 17, 2627–2634;
- 4bJ. S. Harvey, V. Gouverneur, Chem. Commun. 2010, 46, 7477–7485;
- 4cI. Wauters, W. Debrouwer, C. V. Stevens, Beilstein J. Org. Chem. 2014, 10, 1064–1096;
- 4dJ. Diesel, N. Cramer, ACS Catal. 2019, 9, 9164–9177;
- 4eS. Lemouzy, L. Giordano, D. Hérault, G. Buono, Eur. J. Org. Chem. 2020, 3351–3366;
- 4fD. S. Glueck, Synlett 2021, 32, 875–884;
- 4gX. Ye, L. Peng, X. Bao, C.-H. Tan, H. Wang, Green Synth. Catal. 2021, 2, 6–18.
- 5For selected examples, see:
- 5aG. Nishida, K. Noguchi, M. Hirano, K. Tanaka, Angew. Chem. Int. Ed. 2008, 47, 3410–3413; Angew. Chem. 2008, 120, 3458–3461;
- 5bJ. S. Harvey, S. J. Malcolmson, K. S. Dunne, S. J. Meek, A. L. Thompson, R. R. Schrock, A. H. Hoveyda, V. Gouverneur, Angew. Chem. Int. Ed. 2009, 48, 762–766; Angew. Chem. 2009, 121, 776–780;
- 5cZ.-Q. Lin, W.-Z. Wang, S.-B. Yan, W.-L. Duan, Angew. Chem. Int. Ed. 2015, 54, 6265–6269; Angew. Chem. 2015, 127, 6363–6367;
- 5dZ.-J. Du, J. Guan, G.-J. Wu, P. Xu, L.-X. Gao, F.-S. Han, J. Am. Chem. Soc. 2015, 137, 632–635;
- 5eZ. Huang, X. Huang, B. Li, C. Mou, S. Yang, B.-A. Song, Y. R. Chi, J. Am. Chem. Soc. 2016, 138, 7524–7527;
- 5fB. Pérez-Saavedra, N. Vazquez-Galinanes, C. Saa, M. Fananas-Mastral, ACS Catal. 2017, 7, 6104–6109;
- 5gY.-S. Jang, M. Dieckmann, N. Cramer, Angew. Chem. Int. Ed. 2017, 56, 15088–15092; Angew. Chem. 2017, 129, 15284–15288;
- 5hY. Sun, N. Cramer, Angew. Chem. Int. Ed. 2017, 56, 364–367; Angew. Chem. 2017, 129, 370–373;
- 5iY.-S. Jang, L. Wozniak, J. Pedroni, N. Cramer, Angew. Chem. Int. Ed. 2018, 57, 12901–12905; Angew. Chem. 2018, 130, 13083–13087;
- 5jZ. Wang, T. Hayashi, Angew. Chem. Int. Ed. 2018, 57, 1702–1706; Angew. Chem. 2018, 130, 1718–1722;
- 5kY. Zhang, F. Zhang, L. Chen, J. Xu, X. Liu, X. Feng, ACS Catal. 2019, 9, 4834–4840;
- 5lB. M. Trost, S. M. Spohr, A. B. Rolka, C. A. Kalnmals, J. Am. Chem. Soc. 2019, 141, 14098–14103;
- 5mG.-H. Yang, Y. Li, X. Li, J.-P. Cheng, Chem. Sci. 2019, 10, 4322–4327;
- 5nR.-Y. Zhu, L. Chen, X.-S. Hu, F. Zhou, J. Zhou, Chem. Sci. 2020, 11, 97–106.
- 6
- 6aJ. R. Moncarz, N. F. Laritcheva, D. S. Glueck, J. Am. Chem. Soc. 2002, 124, 13356–13357;
- 6bC. Korff, G. Helmchen, Chem. Commun. 2004, 530–531;
- 6cS. Pican, A.-C. Gaumont, Chem. Commun. 2005, 2393–2395;
- 6dV. S. Chan, I. C. Stewart, R. G. Bergman, F. D. Toste, J. Am. Chem. Soc. 2006, 128, 2786–2787;
- 6eC. Scriban, D. S. Glueck, J. Am. Chem. Soc. 2006, 128, 2788–2789;
- 6fN. F. Blank, J. R. Moncarz, T. J. Brunker, C. Scriban, B. J. Anderson, O. Amir, D. S. Glueck, L. N. Zakharov, J. A. Golen, C. D. Incarvito, A. L. Rheingold, J. Am. Chem. Soc. 2007, 129, 6847–6858;
- 6gV. S. Chan, R. G. Bergman, F. D. Toste, J. Am. Chem. Soc. 2007, 129, 15122–15123;
- 6hT. J. Brunker, B. J. Anderson, N. F. Blank, D. S. Glueck, A. L. Rheingold, Org. Lett. 2007, 9, 1109–1112;
- 6iB. J. Anderson, M. A. Guino-o, D. S. Glueck, J. A. Golen, A. G. DiPasquale, L. M. Liable-Sands, A. L. Rheingold, Org. Lett. 2008, 10, 4425–4428;
- 6jV. S. Chan, M. Chiu, R. G. Bergman, F. D. Toste, J. Am. Chem. Soc. 2009, 131, 6021–6032;
- 6kS. K. Gibbons, Z. Xu, R. P. Hughes, S. D. Glueck, A. L. Rheingold, Organometallics 2018, 37, 2159–2166;
- 6lS. Zhang, J.-Z. Xiao, Y.-B. Li, C.-Y. Shi, L. Yin, J. Am. Chem. Soc. 2021, 143, 9912–9921.
- 7
- 7aY. Zhang, H. He, Q. Wang, Q. Cai, Tetrahedron Lett. 2016, 57, 5308–5311;
- 7bR. Beaud, R. J. Phipps, M. J. Gaunt, J. Am. Chem. Soc. 2016, 138, 13183–13186;
- 7cX.-T. Liu, Y.-Q. Zhang, X.-Y. Han, S.-P. Sun, Q.-W. Zhang, J. Am. Chem. Soc. 2019, 141, 16584–16589;
- 7dQ. Dai, W. Li, Z. Li, J. Zhang, J. Am. Chem. Soc. 2019, 141, 20556–20564.
- 8
- 8aX. Fu, W.-T. Loh, Y. Zhang, T. Chen, T. Ma, H. Liu, J. Wang, C.-H. Tan, Angew. Chem. Int. Ed. 2009, 48, 7387–7390; Angew. Chem. 2009, 121, 7523–7526;
- 8bS. Liu, Z. Zhang, F. Xie, N. A. Butt, L. Sun, W. Zhang, Tetrahedron: Asymmetry 2012, 23, 329–332;
- 8cY. Toda, M. Pink, J. N. Johnston, J. Am. Chem. Soc. 2014, 136, 14734–14737;
- 8dJ.-P. Wang, S.-Z. Nie, Z.-Y. Zhou, J.-J. Ye, J.-H. Wen, C.-Q. Zhao, J. Org. Chem. 2016, 81, 7644–7653;
- 8eS.-Z. Nie, R. T. Davison, V. M. Dong, J. Am. Chem. Soc. 2018, 140, 16450–16454;
- 8fM. Wang, L. Zhang, X. Huo, Z. Zhang, Q. Yuan, P. Li, J. Chen, Y. Zou, Z. Wu, W. Zhang, Angew. Chem. Int. Ed. 2020, 59, 20814–20819; Angew. Chem. 2020, 132, 21000–21005.
- 9
- 9aY. Huang, S. A. Pullarkat, S. Teong, R. J. Chew, Y. Li, P.-H. Leung, Organometallics 2012, 31, 4871–4875;
- 9bY. Huang, Y. Li, P.-H. Leung, T. Hayashi, J. Am. Chem. Soc. 2014, 136, 4865–4868;
- 9cC. Li, Q.-L. Bian, S. Xu, W.-L. Duan, Org. Chem. Front. 2014, 1, 541–545;
- 9dL. B. Balázs, Y. Huang, J. B. Khalikuzzaman, Y. Li, S. A. Pullarkat, P.-H. Leung, J. Org. Chem. 2020, 85, 14763–14771.
- 10
- 10aI. Kovacik, D. K. Wicht, N. S. Grewal, D. S. Glueck, C. D. Incarvito, I. A. Guzei, A. L. Rheingold, Organometallics 2000, 19, 950–953;
- 10bC. Scriban, I. Kovacik, D. S. Glueck, Organometallics 2005, 24, 4871–4874;
- 10cB. Join, D. Mimeau, O. Delacroix, A.-C. Gaumont, Chem. Commun. 2006, 3249–3251;
- 10dY. Huang, S. A. Pullarkat, Y. Li, P.-H. Leung, Inorg. Chem. 2012, 51, 2533–2540;
- 10eW.-J. Yue, J.-Z. Xiao, S. Zhang, L. Yin, Angew. Chem. Int. Ed. 2020, 59, 7057–7062; Angew. Chem. 2020, 132, 7123–7128;
- 10fY.-B. Li, H. Tian, L. Yin, J. Am. Chem. Soc. 2020, 142, 20098–20106.
- 11
- 11aQ. Dai, L. Liu, Y. Qian, W. Li, J. Zhang, Angew. Chem. Int. Ed. 2020, 59, 20645–20650; Angew. Chem. 2020, 132, 20826–20831;
- 11bX.-T. Liu, X.-Y. Han, Y. Wu, Y.-Y. Sun, L. Gao, Z. Huang, Q.-W. Zhang, J. Am. Chem. Soc. 2021, 143, 11309–11316.
- 12
- 12aA. Ohashi, S.-I. Kikuchi, M. Yasutake, T. Imamoto, Eur. J. Org. Chem. 2002, 2535–2546;
- 12bM. Stankevic, G. Andrijewski, K. M. Pietrusiewicz, Synlett 2004, 311–315;
- 12cT. Imamoto, K. Sugita, K. Yoshida, J. Am. Chem. Soc. 2005, 127, 11934–11935;
- 12dM. Stankević, K. M. Pietrusiewicz, J. Org. Chem. 2007, 72, 816–822;
- 12eD. Gatineau, L. Giordano, G. Buono, J. Am. Chem. Soc. 2011, 133, 10728–10731;
- 12fZ. Yang, D. Liu, Y. Liu, M. Sugiya, T. Imamoto, W. Zhang, Organometallics 2015, 34, 1228–1237;
- 12gA. O. Kolodiazhna, O. I. Kolodiazhnyi, Symmetry 2020, 12, 108–159.
- 13
- 13aA. Bader, T. Nullmeyers, M. Pabel, G. Salem, A. C. Willis, S. B. Wild, Inorg. Chem. 1995, 34, 384–389;
- 13bF. A. Kortmann, M.-C. Chang, E. Otten, E. P. A. Couzijn, M. Lutz, A. J. Minnaard, Chem. Sci. 2014, 5, 1322–1327.
- 14
- 14aA. Bader, M. Pabel, S. B. Wild, J. Chem. Soc. Chem. Commun. 1994, 30, 1405–1406;
- 14bJ. Drabowicz, P. Łyzwa, J. Omelanczuk, K. M. Pietrusiewicz, M. Mikołajczyk, Tetrahedron: Asymmetry 1999, 10, 2757–2763;
- 14cA. Leyris, J. Bigeault, D. Nuel, L. Giordano, G. Buono, Tetrahedron Lett. 2007, 48, 5247–5250;
- 14dZ. S. Han, H. Wu, Y. Xu, Y. Zhang, B. Qu, Z. Li, D. R. Caldwell, K. R. Fandrick, L. Zhang, F. Roschangar, J. J. Song, C. H. Senanayake, Org. Lett. 2017, 19, 1796–1799;
- 14eS.-G. Li, M. Yuan, F. Topic, Z. S. Han, C. H. Senanayake, Y. S. Tsantrizos, J. Org. Chem. 2019, 84, 7291–7302; For review, see:
- 14fD. S. Glueck, Synthesis 2021, https://doi.org/10.1055/a-1582-0169.
- 15C. Wang, K. Huang, J. Ye, W.-L. Duan, J. Am. Chem. Soc. 2021, 143, 5685–5690.
- 16
- 16aL. Ackermann, Synthesis 2006, 1557–1571;
- 16bA. Christiansen, C. Li, M. Garland, D. Selent, R. Ludwig, A. Spannenberg, W. Baumann, R. Franke, A. Börner, Eur. J. Org. Chem. 2010, 2733–2741;
- 16cT. M. Shaikh, C.-M. Weng, F.-E. Hong, Coord. Chem. Rev. 2012, 256, 771–803;
- 16dG. Manca, M. Caporali, A. Ienco, M. Peruzzini, C. Mealli, J. Organomet. Chem. 2014, 760, 177–185;
- 16eA. Gallen, A. Riera, X. Verdaguer, A. Grabulosa, Catal. Sci. Technol. 2019, 9, 5504–5561.
- 17H. Qiu, Q. Dai, J. He, W. Li, J. Zhang, Chem. Sci. 2020, 11, 9983–9988.
- 18H. Narahashi, A. Yamamoto, I. Shimizu, Chem. Lett. 2004, 33, 348–349.
- 19Y. Qian, Q. Dai, Z. Li, Y. Liu, J. Zhang, Org. Lett. 2020, 22, 4742–4748.
- 20The absolute configuration of 3 aa was unambiguously determined to be (R) by X-ray crystallographic analysis (CCDC number: 2086540) and those of other products and recovered enantioenriched SPOs were assigned by analogy.
- 21The absolute configuration of 4 at was unambiguously determined to be (R,R) by X-ray crystallographic analysis (CCDC number: 2086541), from which we can infer that the configuration before it was reduced (the absolute configuration of 3 at) was (R,R). (The stereocontrolled reduction method (MeOTf/ LiAlH4 at −70 °C) we used, takes place with inversion of configuration at the P-center).
- 22
- 22aB. S. Williams, P. Dani, M. Lutz, A. L. Spek, G. van Koten, Helv. Chim. Acta 2001, 84, 3519–3530;
- 22bD. Morales-Morales, J. Organomet. Chem. 2002, 654, 44–50;
- 22cJ.-K. Liu, J.-F. Gong, M.-P. Song, Org. Biomol. Chem. 2019, 17, 6069–6098;
- 22dY. Xiang, Q. Ge, S. Wu, X. Zheng, Z. Yang, RSC Adv. 2020, 10, 9563–9578.
- 23
- 23aR. K. Haynes, W. W.-L. Lam, L.-L. Yeung, Tetrahedron Lett. 1996, 37, 4729–4732;
- 23bW. W.-L. Lam, R. K. Haynes, L.-L. Yeung, E. W.-K. Chan, Tetrahedron Lett. 1996, 37, 4733–4736;
- 23cR. K. Haynes, W. W.-L. Lam, I. D. Williams, L.-L. Yeung, Chem. Eur. J. 1997, 3, 2052–2057;
- 23dR. K. Haynes, T.-L. Au-Yeung, W.-K. Chan, W.-L. Lam, Z.-Y. Li, L.-L. Yeung, A. S. C. Chan, P. Li, M. Koen, C. R. Mitchell, S. C. Vonwiller, Eur. J. Org. Chem. 2000, 3205–3216;
- 23eI. N. Francesco, A. Wagner, F. Colobert, Chem. Commun. 2010, 46, 2139–2141.
- 24
- 24aK. Tamura, M. Sugiya, K. Yoshida, A. Yanagisawa, T. Imamoto, Org. Lett. 2010, 12, 4400–4403;
- 24bT. Imamoto, K. Tamura, Z. Zhang, Y. Horiuchi, M. Sugiya, K. Yoshida, A. Yanagisawa, I. D. Gridnev, J. Am. Chem. Soc. 2012, 134, 1754–1769;
- 24cH. Iwamoto, Y. Ozawa, Y. Takenouchi, T. Imamoto, H. Ito, J. Am. Chem. Soc. 2021, 143, 6413–6422.
- 25The exact structure of (R,R)-5 at was determined by X-ray analysis as shown in Scheme 2 (CCDC number: 2106864), which uncovered its absolute configuration.
- 26
- 26aR. J. Chew, P.-H. Leung, Chem. Rec. 2016, 16, 141–158;
- 26bJ.-J. Feng, X.-F. Chen, M. Shi, W.-L. Duan, J. Am. Chem. Soc. 2010, 132, 5562–5563;
- 26cY. Huang, S. A. Pullarkat, Y. Li, P.-H. Leung, Chem. Commun. 2010, 46, 6950–6952.
- 27Since acceptance of this manuscript, a related study was published: Z.-H. Wu, A.-Q. Chen, M. Yuan, Y.-X. Zhao, H.-L. Yang, L.-H. Wei, H.-Y. Wang, T. Wang, Z. Zhang, and W.-L. Duan, Angew. Chem. Int. Ed. 2021, https://doi.org/10.1002/anie.202111137;
Angew. Chem. 2021, https://doi.org/10.1002/ange.202111137.
10.1002/ange.202111137 Google Scholar