Isomerization of a cis-(2-Borylalkenyl)Gold Complex via a Retro-1,2-Metalate Shift: Cleavage of a C−C/C−Si Bond trans to a C−Au Bond
Akane Suzuki
Department of Molecular and Macromolecular Chemistry, Graduate School of Engineering, Nagoya University, Furo-cho, Chikusa-ku, Nagoya, 464-8603 Aichi, Japan
These authors contributed equally to this work.
Search for more papers by this authorLinlin Wu
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, Hong Kong
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhenyang Lin
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, Hong Kong
Search for more papers by this authorCorresponding Author
Prof. Dr. Makoto Yamashita
Department of Molecular and Macromolecular Chemistry, Graduate School of Engineering, Nagoya University, Furo-cho, Chikusa-ku, Nagoya, 464-8603 Aichi, Japan
Search for more papers by this authorAkane Suzuki
Department of Molecular and Macromolecular Chemistry, Graduate School of Engineering, Nagoya University, Furo-cho, Chikusa-ku, Nagoya, 464-8603 Aichi, Japan
These authors contributed equally to this work.
Search for more papers by this authorLinlin Wu
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, Hong Kong
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhenyang Lin
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, Hong Kong
Search for more papers by this authorCorresponding Author
Prof. Dr. Makoto Yamashita
Department of Molecular and Macromolecular Chemistry, Graduate School of Engineering, Nagoya University, Furo-cho, Chikusa-ku, Nagoya, 464-8603 Aichi, Japan
Search for more papers by this authorGraphical Abstract
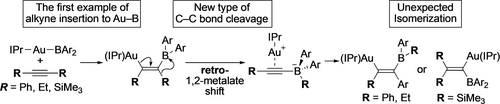
Unexpected isomerization of cis-2-borylalkenylgold complexes, which formed through the first alkyne insertion to a Au−B bond, was observed with structural characterization of intermediates and products. Kinetic analysis for isomerization of isolated intermediate and detailed DFT calculations revealed a new elementary reaction, i.e., a “retro-1,2-metalate shift” that contains anti-β-carbon/silicon elimination.
Abstract
This manuscript describes the first example of an alkyne insertion to the Au−B bond of a di(o-tolyl)borylgold complex to afford a cis-2-borylalkenylgold complex, and its isomerization to result in interchanging substituents on the alkenyl carbon atom and the boron atom. The former reaction is the first example of an alkyne insertion to a Au−B bond. In the latter reaction, the regiochemistry of the isomerized alkenylgold products varied depending on the substituents. DFT calculations revealed the formation of gold alkynylborates as a common intermediate via a “retro-1,2-metalate shift”, which can be considered as an anti-β-carbon/silicon elimination, and identified a subsequent 1,2-metalate shift as the regiochemistry-determining step. Relative energies of the transition states to each isomer and natural-bond-orbital (NBO) analyses were used to clearly rationalize the regiochemistry of the products.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202108530-sup-0001-misc_information.pdf2.9 MB | Supporting Information |
| anie202108530-sup-0001-SI.cif6.6 MB | Supporting Information |
| anie202108530-sup-0001-SI.xyz192.3 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1The first issue of Chem. Rev. in 2021 focuses on the “Carbon–Carbon Bond Cleavage in Stereoselective Synthesis” to show the importance of C−C bond cleavage.
- 2
- 2aM. D. R. Lutz, B. Morandi, Chem. Rev. 2021, 121, 300–326;
- 2bB. Wang, M. A. Perea, R. Sarpong, Angew. Chem. Int. Ed. 2020, 59, 18898–18919; Angew. Chem. 2020, 132, 19058–19080;
- 2cP. Sivaguru, Z. Wang, G. Zanoni, X. Bi, Chem. Soc. Rev. 2019, 48, 2615–2656.
- 3
- 3aT. R. McDonald, L. R. Mills, M. S. West, S. A. L. Rousseaux, Chem. Rev. 2021, 121, 3–79;
- 3bO. O. Sokolova, J. F. Bower, Chem. Rev. 2021, 121, 80–109;
- 3cJ. Wang, S. A. Blaszczyk, X. Li, W. Tang, Chem. Rev. 2021, 121, 110–139;
- 3dY. Cohen, A. Cohen, I. Marek, Chem. Rev. 2021, 121, 140–161;
- 3eR. Vicente, Chem. Rev. 2021, 121, 162–226;
- 3fV. Pirenne, B. Muriel, J. Waser, Chem. Rev. 2021, 121, 227–263;
- 3gM. Murakami, N. Ishida, Chem. Rev. 2021, 121, 264–299.
- 4K. Nogi, H. Yorimitsu, Chem. Rev. 2021, 121, 345–364.
- 5
- 5aD. P. Smith, J. R. Strickler, S. D. Gray, M. A. Bruck, R. S. Holmes, D. E. Wigley, Organometallics 1992, 11, 1275–1288.;
- 5bW. Ma, C. Yu, T. Chen, L. Xu, W.-X. Zhang, Z. Xi, Chem. Soc. Rev. 2017, 46, 1160–1192.;
- 5cV. H. Gessner, J. F. Tannaci, A. D. Miller, T. D. Tilley, Acc. Chem. Res. 2011, 44, 435–446..
- 6
- 6aP. Zhao, J. F. Hartwig, J. Am. Chem. Soc. 2005, 127, 11618–11619;
- 6bP. Zhao, J. F. Hartwig, Organometallics 2008, 27, 4749–4757;
- 6cY. Ikeda, Y. Mutoh, K. Imai, N. Tsuchida, K. Takano, Y. Ishii, Organometallics 2013, 32, 4353–4358.
- 7
- 7aG. J. Irvine, M. J. G. Lesley, T. B. Marder, N. C. Norman, C. R. Rice, E. G. Robins, W. R. Roper, G. R. Whittell, L. J. Wright, Chem. Rev. 1998, 98, 2685–2722;
- 7bH. Braunschweig, M. Colling, Coord. Chem. Rev. 2001, 223, 1–51;
- 7cS. Aldridge, D. L. Coombs, Coord. Chem. Rev. 2004, 248, 535–559;
- 7d“Transition metal boryl complexes”: D. L. Kays, S. Aldridge, Contemporary Metal Boron Chemistry I: Borylenes, Boryls, Borane, Vol. 130, Springer, Heidelberg, 2008, pp. 29–122.
- 8
- 8aD. Hemming, R. Fritzemeier, S. A. Westcott, W. L. Santos, P. G. Steel, Chem. Soc. Rev. 2018, 47, 7477–7494;
- 8bX. Guo, T. Yang, F. K. Sheong, Z. Lin, ACS Catalysis 2021, 11, 5061–5068.
- 9
- 9aY. Segawa, M. Yamashita, K. Nozaki, Angew. Chem. Int. Ed. 2007, 46, 6710–6713; Angew. Chem. 2007, 119, 6830–6833;
- 9bW. Lu, H. Hu, Y. Li, R. Ganguly, R. Kinjo, J. Am. Chem. Soc. 2016, 138, 6650–6661;
- 9cH. Niu, R. J. Mangan, A. V. Protchenko, N. Phillips, W. Unkrig, C. Friedmann, E. L. Kolychev, R. Tirfoin, J. Hicks, S. Aldridge, Dalton Trans. 2018, 47, 7445–7455;
- 9dC. M. Zinser, F. Nahra, L. Falivene, M. Brill, D. B. Cordes, A. M. Z. Slawin, L. Cavallo, C. S. J. Cazin, S. P. Nolan, Chem. Commun. 2019, 55, 6799–6802;
- 9eA. Suzuki, X. Guo, Z. Lin, M. Yamashita, Chem. Sci. 2021, 12, 917–928.
- 10
- 10aY. Segawa, M. Yamashita, K. Nozaki, Science 2006, 314, 113–115;
- 10bM. Yamashita, Y. Suzuki, Y. Segawa, K. Nozaki, J. Am. Chem. Soc. 2007, 129, 9570–9571;
- 10cM. Yamashita, Y. Suzuki, Y. Segawa, K. Nozaki, Chem. Lett. 2008, 37, 802–803;
- 10dM. Yamashita, Bull. Chem. Soc. Jpn. 2011, 84, 983–999;
- 10e“Boryl Anions”: M. Yamashita, K. Nozaki in Synthesis and Application of Organoboron Compounds (Eds.: E. Fernández, A. Whiting), Springer International Publishing, Cham, 2015, pp. 1–37.
10.1007/978-3-319-13054-5_1 Google Scholar
- 11
- 11aK. K. Das, S. Panda, Chem. Eur. J. 2020, 26, 14270–14282;
- 11bH. Wang, C. Jing, A. Noble, V. K. Aggarwal, Angew. Chem. Int. Ed. 2020, 59, 16859–16872; Angew. Chem. 2020, 132, 17005–17018;
- 11cS. Namirembe, J. P. Morken, Chem. Soc. Rev. 2019, 48, 3464–3474;
- 11dD. S. Matteson, Chem. Rev. 1989, 89, 1535–1551.
- 12
- 12aH. C. Brown, B. C. S. Rao, J. Am. Chem. Soc. 1956, 78, 5694–5695;
- 12bM. E. D. Hillman, J. Am. Chem. Soc. 1962, 84, 4715–4720.
- 13
- 13aG. Kehr, G. Erker, Chem. Sci. 2016, 7, 56–65;
- 13bG. Kehr, G. Erker, Chem. Commun. 2012, 48, 1839–1850;
- 13cB. Wrackmeyer, Coord. Chem. Rev. 1995, 145, 125–156;
- 13dB. Wrackmeyer, K. Horchler, Organometallics 1990, 9, 1881–1886;
- 13eB. Wrackmeyer, Organometallics 1984, 3, 1–4.
- 14
- 14aP. Binger, R. Köster, Tetrahedron Lett. 1965, 6, 1901–1906;
10.1016/S0040-4039(01)83885-5 Google Scholar
- 14bP. Binger, R. Köster, Synthesis 1973, 309–311;
- 14cP. Binger, R. Köster, J. Organomet. Chem. 1974, 73, 205–210;
- 14dP. Binger, R. Köster, Synthesis 1974, 350–351;
- 14eA. Pelter, C. Subrahmanyam, R. J. Laub, K. J. Gould, C. R. Harrison, Tetrahedron Lett. 1975, 16, 1633–1636;
10.1016/S0040-4039(00)72218-0 Google Scholar
- 14fA. Pelter, K. J. Gould, C. R. Harrison, Tetrahedron Lett. 1975, 16, 3327–3330;
10.1016/S0040-4039(00)91440-0 Google Scholar
- 14gA. Pelter, T. W. Bentley, C. R. Harrison, C. Subrahmanyam, R. J. Laub, J. Chem. Soc. Perkin Trans. 1 1976, 2419–2428;
- 14hA. Pelter, K. J. Gould, C. R. Harrison, J. Chem. Soc. Perkin Trans. 1 1976, 2428–2434;
- 14iJ. Hooz, R. Mortimer, Tetrahedron Lett. 1976, 17, 805–808;
10.1016/S0040-4039(00)92889-2 Google Scholar
- 14jR. Koster, Pure Appl. Chem. 1977, 49, 765–789;
- 14kJ. Hooz, R. D. Mortimer, Can. J. Chem. 1978, 56, 2786–2788;
- 14lA. Pelter, M. E. Colclough, Tetrahedron Lett. 1986, 27, 1935–1938;
- 14mA. Pelter, M. Eamon Colclough, Tetrahedron 1995, 51, 811–828;
- 14nJ. Gerard, L. Hevesi, Tetrahedron 2001, 57, 9109–9121.
- 15H. Ye, Z. Lu, D. You, Z. Chen, Z. H. Li, H. Wang, Angew. Chem. Int. Ed. 2012, 51, 12047–12050; Angew. Chem. 2012, 124, 12213–12216.
- 16C. A. Theulier, Y. García-Rodeja, N. Saffon-Merceron, K. Miqueu, G. Bouhadir, D. Bourissou, Chem. Commun. 2021, 57, 347–350.
- 17It should be noted that a migratory insertion of unsaturated bond to Au−Si bond has also been rarely observed. See:
- 17aM. Joost, P. Gualco, S. Mallet-Ladeira, A. Amgoune, D. Bourissou, Angew. Chem. Int. Ed. 2013, 52, 7160–7163; Angew. Chem. 2013, 125, 7301–7304;
- 17bM. Joost, L. Estevez, S. Mallet-Ladeira, K. Miqueu, A. Amgoune, D. Bourissou, J. Am. Chem. Soc. 2014, 136, 10373–10382. In contrast to that the reported insertion of phenylacetylene and ethyl 2,3-butadienoate to Au−Si bond requires 60 °C to proceed, the present alkyne insertion to Au−B bond takes place smoothly at room temperature even the substrates are internal alkynes.
- 18J. Emsley, The Elements, 3rd ed., Oxford University Press, New York, 1998.
- 19N. Phillips, R. Kong, A. White, M. R. Crimmin, Angew. Chem. Int. Ed. 2021, 60, 12013–12019; Angew. Chem. 2021, 133, 12120–12126.
- 20Zwitterionic imidazolium borataallene has been reported; for details, see: R. Bertermann, H. Braunschweig, C. K. L. Brown, A. Damme, R. D. Dewhurst, C. Horl, T. Kramer, I. Krummenacher, B. Pfaffinger, K. Radacki, Chem. Commun. 2014, 50, 97–99.
- 21C. Kojima, K.-H. Lee, Z. Lin, M. Yamashita, J. Am. Chem. Soc. 2016, 138, 6662–6669.
- 22M. M. Hansmann, F. Rominger, M. P. Boone, D. W. Stephan, A. S. K. Hashmi, Organometallics 2014, 33, 4461–4470.
- 23G. Klatt, R. Xu, M. Pernpointner, L. Molinari, T. Quang Hung, F. Rominger, A. S. K. Hashmi, H. Köppel, Chem. Eur. J. 2013, 19, 3954–3961.
- 24It should be noted that 6-Me(IMe) in Scheme 5 or 6-Si(IMe) in Scheme 6 in the potential energy profiles represents a series of very-close-in-energy rotamers (with respect to the relative orientation of the NHC ligand and the borate moiety), which are able to switch from one to another easily. In the Figure, we take the lowest energy species as the representative.
- 25Z. Wang, Y. Zhou, J. X. Zhang, I. Krummenacher, H. Braunschweig, Z. Lin, Chem. Eur. J. 2018, 24, 9612–9621.
- 26
- 26aD. Schneider, H. Werner, Angew. Chem. Int. Ed. Engl. 1991, 30, 700–702; Angew. Chem. 1991, 103, 710–712;
- 26bH. Werner, M. Baum, D. Schneider, B. Windmueller, Organometallics 1994, 13, 1089–1097;
- 26cN. G. Connelly, W. E. Geiger, C. Lagunas, B. Metz, A. L. Rieger, P. H. Rieger, M. J. Shaw, J. Am. Chem. Soc. 1995, 117, 12202–12208;
- 26dK. Onitsuka, H. Katayama, K. Sonogashira, F. Ozawa, J. Chem. Soc. Chem. Commun. 1995, 2267–2268;
- 26eH. Katayama, K. Onitsuka, F. Ozawa, Organometallics 1996, 15, 4642–4645;
- 26fH. Werner, R. W. Lass, O. Gevert, J. Wolf, Organometallics 1997, 16, 4077–4088;
- 26gH. Katayama, F. Ozawa, Organometallics 1998, 17, 5190–5196;
- 26hD. Huang, W. E. Streib, O. Eisenstein, K. G. Caulton, Organometallics 2000, 19, 1967–1972;
- 26iH. Katayama, C. Wada, K. Taniguchi, F. Ozawa, Organometallics 2002, 21, 3285–3291;
- 26jM. V. Jiménez, E. Sola, F. J. Lahoz, L. A. Oro, Organometallics 2005, 24, 2722–2729;
- 26kK. Ilg, M. Paneque, M. L. Poveda, N. Rendón, L. L. Santos, E. Carmona, K. Mereiter, Organometallics 2006, 25, 2230–2236;
- 26lM. Konkol, D. Steinborn, J. Organomet. Chem. 2006, 691, 2839–2845;
- 26mR. W. Lass, H. Werner, Inorg. Chim. Acta 2011, 369, 288–291;
- 26nC.-F. Yeung, L.-H. Chung, H.-S. Lo, C.-H. Chiu, J. Cai, C.-Y. Wong, Organometallics 2015, 34, 1963–1968.
- 27Z. Wang, Y. Zhou, T. B. Marder, Z. Lin, Org. Biomol. Chem. 2017, 15, 7019–7027.
- 28
- 28aS. G. Curto, M. A. Esteruelas, M. Oliván, E. Oñate, A. Vélez, Organometallics 2018, 37, 1970–1978;
- 28bS. G. Curto, M. A. Esteruelas, M. Oliván, E. Oñate, Organometallics 2019, 38, 4183–4192.
- 29A. Yamamoto, M. Suginome, J. Am. Chem. Soc. 2005, 127, 15706–15707.
- 30J. V. Obligacion, J. M. Neely, A. N. Yazdani, I. Pappas, P. J. Chirik, J. Am. Chem. Soc. 2015, 137, 5855–5858.
- 31M. P. Boone, D. W. Stephan, Organometallics 2011, 30, 5537–5542.
- 32Deposition Number(s) 2078484 (for 2-Ph), 2078485 (for 2-Et), 2078486 (for 3-Et), 2078487 (for 4-Si), and 2078488 (for 5-Si) contain(s) the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.