Bioinspired Network Analysis Enabled Divergent Syntheses and Structure Revision of Pentacyclic Cytochalasans
Hai Wu
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, 650201 China
University of Chinese Academy of Sciences, Beijing, 100049 China
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
These authors contributed equally to this work.
Search for more papers by this authorYiming Ding
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, 650201 China
University of Chinese Academy of Sciences, Beijing, 100049 China
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
These authors contributed equally to this work.
Search for more papers by this authorKun Hu
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, 650201 China
These authors contributed equally to this work.
Search for more papers by this authorXianwen Long
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, 650201 China
University of Chinese Academy of Sciences, Beijing, 100049 China
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorChunlei Qu
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, 650201 China
University of Chinese Academy of Sciences, Beijing, 100049 China
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Pema-Tenzin Puno
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, 650201 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Jun Deng
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, 650201 China
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorHai Wu
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, 650201 China
University of Chinese Academy of Sciences, Beijing, 100049 China
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
These authors contributed equally to this work.
Search for more papers by this authorYiming Ding
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, 650201 China
University of Chinese Academy of Sciences, Beijing, 100049 China
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
These authors contributed equally to this work.
Search for more papers by this authorKun Hu
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, 650201 China
These authors contributed equally to this work.
Search for more papers by this authorXianwen Long
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, 650201 China
University of Chinese Academy of Sciences, Beijing, 100049 China
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorChunlei Qu
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, 650201 China
University of Chinese Academy of Sciences, Beijing, 100049 China
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Pema-Tenzin Puno
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, 650201 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Jun Deng
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, 650201 China
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorDedicated to the 100th anniversary of Chemistry at Nankai University
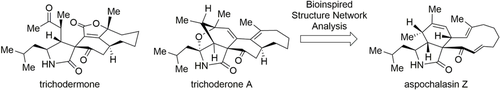
Graphical Abstract
The bioinspired, divergent total syntheses of trichodermone and trichoderone A were accomplished from a common biogenetic precursor, aspochalasin Z. Key steps include transannular alkene cyclizations, a singlet oxygen ene reaction, and hydrogen atom transfer (HAT) cascade reactions. This approach validates the proposed biosynthetic pathway from a chemical perspective and paves the way for the synthesis and characterization of other cytochalasans.
Abstract
We accomplished the divergent total syntheses of ten pentacyclic cytochalasans (aspergillin PZ, trichodermone, trichoderones, flavipesines, and flavichalasines) from a common precursor aspochalasin D and revised the structures of trichoderone B, spicochalasin A, flavichalasine C, aspergilluchalasin based on structure network analysis of the cytochalasans biosynthetic pathways and DFT calculations. The key steps of the syntheses include transannular alkene/epoxyalkene and carbonyl-ene cyclizations to establish the C/D ring of pentacyclic aspochalasans. Our bioinspired approach to these pentacyclic cytochalasans validate the proposed biosynthetic speculation from a chemical view and provide a platform for the synthesis of more than 400 valuable cytochalasans bearing different macrocycles and amino-acid residues.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202102831-sup-0001-misc_information.pdf11.8 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. Clardy, C. Walsh, Nature 2004, 432, 829–837;
- 1bP. A. Wender, B. L. Miller, Nature 2009, 460, 197–201.
- 2For leading reviews on cytochalasans, see:
- 2aE. Skellam, Nat. Prod. Rep. 2017, 34, 1252–1263;
- 2bK. Scherlach, D. Boettger, N. Remme, C. Hertweck, Nat. Prod. Rep. 2010, 27, 869–886; for the isolation of tricyclic cytochalasans related with this study, see:
- 2cK. S. Walter, K. Ernst, Helv. Chim. Acta 1979, 62, 1501–1524;
- 2dT. Tomikawa, K. Shin-ya, T. Kinoshita, A. Miyajima, H. Seto, Y. Hayakawa, J. Antibiot. 2001, 54, 379–381.
- 3For syntheses of cytochalasans:
- 3aG. Stork, Y. Nakahara, Y. Nakahara, W. J. Greenlee, J. Am. Chem. Soc. 1978, 100, 7775–7777;
- 3bG. Stork, E. Nakamura, J. Am. Chem. Soc. 1983, 105, 5510–5512;
- 3cB. M. Trost, M. Ohmori, S. A. Boyd, H. Okawara, S. J. Brickner, J. Am. Chem. Soc. 1989, 111, 8281–8284;
- 3dE. J. Thomas, J. W. F. Whitehead, J. Chem. Soc. Perkin Trans. 1 1989, 507–518;
- 3eH. Dyke, R. Sauter, P. Steel, E. J. Thomas, J. Chem. Soc. Chem. Commun. 1986, 1447–1449;
- 3fE. J. Thomas, J. W. F. Whitehead, J. Chem. Soc. Chem. Commun. 1986, 727–728;
- 3gE. Merifield, E. J. Thomas, J. Chem. Soc. Chem. Commun. 1990, 464–466;
- 3hE. Merifield, E. J. Thomas, J. Chem. Soc. Perkin Trans. 1 1999, 3269–3283;
- 3iE. Vedejs, S. M. Duncan, J. Org. Chem. 2000, 65, 6073–6081;
- 3jE. Vedejs, J. G. Reid, J. D. Rodgers, S. J. Wittenberger, J. Am. Chem. Soc. 1990, 112, 4351–4357;
- 3kJ. Auerbach, S. M. Weinreb, J. Org. Chem. 1975, 40, 3311–3312;
- 3lM. J. Hensel, J. T. Palmer, K. S. Learn, P. L. Fuchs, Synth. Commun. 1986, 16, 1297–1314;
- 3mM. Boutellier, D. Wallach, C. Tamm, Helv. Chim. Acta 1993, 76, 2515–2527;
- 3nA. M. Haidle, A. G. Myers, Proc. Natl. Acad. Sci. USA 2004, 101, 12048–12053;
- 3oM. Zaghouani, C. Kunz, L. Guédon, F. Blanchard, B. Nay, Chem. Eur. J. 2016, 22, 15257–15260;
- 3pZ. Zeng, C. Chen, Y. Zhang, Org. Chem. Front. 2018, 5, 838–840.
- 4
- 4aB.-C. Yan, W.-G. Wang, D.-B. Hu, X. Sun, L.-M. Kong, X.-N. Li, X. Du, S.-H. Luo, Y. Liu, Y. Li, H.-D. Sun, J.-X. Pu, Org. Lett. 2016, 18, 1108–1111;
- 4bM.-H. Yang, M.-L. Gu, C. Han, X.-J. Guo, G.-P. Yin, P. Yu, L.-Y. Kong, Org. Lett. 2018, 20, 3345–3348;
- 4cW.-X. Wang, Z.-H. Li, T. Feng, J. Li, H. Sun, R. Huang, Q.-X. Yuan, H.-L. Ai, J.-K. Liu, Org. Lett. 2018, 20, 7758–7761;
- 4dW.-X. Wang, X. Lei, H.-L. Ai, X. Bai, J. Li, J. He, Z.-H. Li, Y.-S. Zheng, T. Feng, J.-K. Liu, Org. Lett. 2019, 21, 1108–1111;
- 4eH.-H. Wang, G. Li, Y.-N. Qiao, Y. Sun, X.-P. Peng, H.-X. Lou, Org. Lett. 2019, 21, 3319–3322;
- 4fW.-X. Wang, X. Lei, Y.-L. Yang, Z.-H. Li, H.-L. Ai, J. Li, T. Feng, J.-K. Liu, Org. Lett. 2019, 21, 6957–6960.
- 5
- 5aS. B. Carter, Nature 1967, 213, 1667–1676;
- 5bS. B. Brown, J. A. Spudich, J. Cell Biol. 1981, 88, 487–491.
- 6
- 6aK. Chen, P. S. Baran, Nature 2009, 459, 824–828;
- 6bA. Mendoza, Y. Ishihara, P. S. Baran, Nat. Chem. 2011, 4, 21–25;
- 6cL. Jørgensen, S. J. Mckerrall, C. A. Kuttruff, F. Ungeheuer, J. Felding, P. S. Baran, Science 2013, 341, 878–882;
- 6dS. Kawamura, H. Chu, J. Felding, P. S. Baran, Nature 2016, 532, 90–93.
- 7N. Shionozaki, N. Iwamura, R. Tanaka, K. Makino, H. Uchiro, Chem. Asian J. 2013, 8, 1243–1252.
- 8E. Thomas, M. Willis, Org. Biomol. Chem. 2014, 12, 7537–7550.
- 9C. Tian, X. Lei, Y. Wang, Z. Dong, G. Liu, Y. Tang, Angew. Chem. Int. Ed. 2016, 55, 6992–6996; Angew. Chem. 2016, 128, 7106–7110.
- 10
- 10aS. M. Canham, L. E. Overmann, P. S. Tanis, Tetrahedron 2011, 67, 9837–9843;
- 10bJ. R. Reyes, N. Winter, L. Spessert, D. Trauner, Angew. Chem. Int. Ed. 2018, 57, 15587–15591; Angew. Chem. 2018, 130, 15813–15817.
- 11Y. Zhang, Y. Pei, H. Hua, B. Feng, J. Antibiot. 2002, 55, 693–695.
- 12G. Ding, H. Wang, L. Li, B. Song, H. Chen, H. Zhang, X. Liu, Z. Zou, J. Nat. Prod. 2014, 77, 164–167.
- 13G. Ding, H. Wang, L. Li, A. J. Chen, L. Chen, H. Chen, H. Zhang, X. Liu, Z. Zou, Eur. J. Org. Chem. 2012, 2516–2519.
- 14X. Zhang, L. Yang, W. Wang, Z. Wu, J. Wang, W. Sun, X. Li, C. Chen, H. Zhu, Y. Zhang, J. Nat. Prod. 2019, 82, 2994–3001.
- 15G. Wei, D. Tan, C. Chen, Q. Tong, X. Li, J. Huang, J. Liu, Y. Xue, J. Wang, Z. Luo, H. Zhu, Y. Zhang, Sci. Rep. 2017, 7, 42434–42445.
- 16
- 16aX. Long, H. Wu, Y. Ding, C. Qu, J. Deng, Chem 2021, 7, 212–223;
- 16bH. Zhu, C. Chen, Y. Xue, Q. Tong, X. Li, X. Chen, J. Wang, G. Yao, Z. Luo, Y. Zhang, Angew. Chem. Int. Ed. 2015, 54, 13374–13378; Angew. Chem. 2015, 127, 13572–13576;
- 16cH. Zhu, C. Chen, Q. Tong, J. Yang, G. Wei, Y. Xue, J. Wang, Z. Luo, Y. Zhang, Angew. Chem. Int. Ed. 2016, 55, 3486–3490; Angew. Chem. 2016, 128, 3547–3551;
- 16dH. Zhu, C. Chen, Q. Tong, X. Li, J. Yang, Y. Xue, Z. Luo, J. Wang, G. Yao, Y. Zhang, Angew. Chem. Int. Ed. 2017, 56, 5242–5246; Angew. Chem. 2017, 129, 5326–5330;
- 16eG. Wei, C. Chen, Q. Tong, J. Huang, W. Wang, Z. Wu, J. Yang, J. Liu, Y. Xue, Z. Luo, J. Wang, H. Zhu, Y. Zhang, Org. Lett. 2017, 19, 4399–4402;
- 16fZ. Wu, Q. Tong, X. Zhang, P. Zhou, C. Dai, J. Wang, C. Chen, H. Zhu, Y. Zhang, Org. Lett. 2019, 21, 1026–1030.
- 17
- 17aK. Ishiuchi, T. Nakazawa, F. Yagishita, T. Mino, H. Noguchi, K. Hotta, K. Watanabe, J. Am. Chem. Soc. 2013, 135, 7371–7377;
- 17bY. Hu, D. Dietrich, W. Xu, A. Patel, J. A. J. Thuss, J. Wang, W.-B. Yin, K. Qiao, W.-B. Houk, J. C. Vederas, Y. Tang, Nat. Chem. Biol. 2014, 10, 552–554;
- 17cC. Wang, V. Hantke, R. J. Cox, E. Skellam, Org. Lett. 2019, 21, 4163–4167;
- 17dC. Wang, K. Becker, S. Pfutze, E. Kuhnert, M. Stadler, R. J. Cox, E. Skellam, Org. Lett. 2019, 21, 8756–8760.
- 18
- 18aE. J. Corey, W. J. Howe, H. W. Orf, D. A. Pensak, G. Petersson, J. Am. Chem. Soc. 1975, 97, 6116–6124;
- 18bE. J. Corey, X. M. Cheng, The Logic of Chemical Synthesis, Wiley, New York, 1995.
- 19
- 19aR. Bao, T. Chong, H. Zhang, Z. Wang, Z. Dong, Y. Li, M. Gao, H. Zhang, G. Liu, Y. Tang, Angew. Chem. Int. Ed. 2018, 57, 14216–14220; Angew. Chem. 2018, 130, 14412–14416;
- 19bX. Long, Y. Ding, J. Deng, Angew. Chem. Int. Ed. 2018, 57, 14221–14224; Angew. Chem. 2018, 130, 14417–14420.
- 20
- 20aS. Inoki, K. Kato, S. Isayama, T. Mukaiyama, Chem. Lett. 1990, 19, 1869–1872;
- 20bY. Y. See, A. T. Herrmann, Y. Aihara, P. S. Baran, J. Am. Chem. Soc. 2015, 137, 13776–13779.
- 21S. Isayama, T. Mukaiyama, Chem. Lett. 1989, 18, 1071–1074.
- 22C. Lei, Z. Yang, Y. Zeng, Y. Zhou, Y. Huang, X. He, G. Li, X. Yuan, Nat. Prod. Res. 2018, 32, 7–13.
- 23X. Long, Y. Ding, H. Wu, J. Deng, Synlett 2020, 31, 301–308.
- 24
- 24aM. Schneider, M. J. R. Richter, E. M. Carreira, J. Am. Chem. Soc. 2020, 142, 17802–17809;
- 24bM. J. R. Richter, M. Schneider, M. Brandstatter, S. Kräutwald, E. M. Carreira, J. Am. Chem. Soc. 2018, 140, 16704–16710;
- 24cL. A. Wein, K. Wurst, P. Angyal, L. Weisheit, T. Magauer, J. Am. Chem. Soc. 2019, 141, 19589–19593.
- 25S. G. Brown, M. J. Jansma, T. R. Hoye, J. Nat. Prod. 2012, 75, 1326–1331.
- 26
- 26aC. Chen, J. Wang, J. Liu, H. Zhu, B. Sun, J. Wang, J. Zhang, Z. Luo, G. Yao, Y. Xue, Y. Zhang, J. Nat. Prod. 2015, 78, 1193–1201;
- 26bW. Wang, J. Gong, X. Liu, C. Dai, Y. Wang, X. Li, J. Wang, Z. Luo, Y. Zhou, Y. Xue, H. Zhu, C. Chen, Y. Zhang, J. Nat. Prod. 2018, 81, 1578–1587.
- 27
- 27aS. Gnaim, J. C. Vantourout, F. Serpier, P. G. Echeverria, P. S. Baran, ACS Catal. 2021, 11, 883–892;
- 27bY. Takahira, H. Wilke, Z. Yao, J. Li, D. Delbrayelle, P. G. Echeverria, J. Vantourout, P. S. Baran, ChemRxiv 2020, https://doi.org/10.26434/chemrxiv.12595340.v1;
- 27cY. Chen, J. P. Romaire, T. R. Newhouse, J. Am. Chem. Soc. 2015, 137, 5875–5878;
- 27dD. Huang, S. M. Szewczyk, P. Zhang, T. R. Newhouse, J. Am. Chem. Soc. 2019, 141, 5669–5674;
- 27eX. Jie, Y. Shang, X. Zhang, W. Su, J. Am. Chem. Soc. 2016, 138, 5623–5633;
- 27fY. Shang, X. Jie, K. Jonnada, S. N. Zafar, W. Su, Nat. Commun. 2017, 8, 2273;
- 27gM. Chen, G. Dong, J. Am. Chem. Soc. 2019, 141, 14889–14897;
- 27hM. Chen, A. J. Rago, G. Dong, Angew. Chem. Int. Ed. 2018, 57, 16205–16209; Angew. Chem. 2018, 130, 16437–16441.
- 28Y. Chen, L. Wu, M. Peng, D. Zhang, L. Zhang, C. Tung, Tetrahedron 2006, 62, 10688–10693.
- 29
- 29aA. J. E. Novak, C. E. Grigglestone, D. Trauner, J. Am. Chem. Soc. 2019, 141, 15515–15518;
- 29bC. Qu, X. Long, Y. Sang, M. Zhang, X. Zhao, X. Xue, J. Deng, Org. Lett. 2020, 22, 9421–9426.
- 30
- 30aK. C. Nicolaou, S. A. Snyder, Angew. Chem. Int. Ed. 2005, 44, 1012–1044; Angew. Chem. 2005, 117, 1036–1069;
- 30bH. Chi, C. J. F. Cole, P. Hu, C. A. Taylor, S. A. Snyder, Chem. Sci. 2020, 11, 10939–10944;
- 30cZ. Meng, A. Fürstner, J. Am. Chem. Soc. 2020, 142, 11703–11708.
- 31
- 31aM. W. Lodewyk, M. R. Siebert, D. J. Tantillo, Chem. Rev. 2012, 112, 1839–1862;
- 31bM. O. Marcarino, M. M. Zanardi, S. Cicetti, A. M. Sarotti, Acc. Chem. Res. 2020, 53, 1922–1932.
- 32L. Chen, Y. Liu, B. Song, H. Zhang, G. Ding, X. Liu, Y. Gu, Z. Zou, Fitoterapia 2014, 96, 115–122.
- 33Z. Lin, T. Zhu, H. Wei, G. Zhang, H. Wang, Q. Gu, Eur. J. Org. Chem. 2009, 3045–3051.
- 34V. Rukachaisirikul, N. Rungsaiwattana, S. Klaiklay, S. Phongpaichit, K. Borwornwiriyapan, J. Sakayaroj, J. Nat. Prod. 2014, 77, 2375–2382.
- 35Deposition Numbers 1921359 (for 2), 1921360 (for 18), 1921361 (for 19), 1921362 (for 9), 2034264 (for 32), 2034265 (for 5), 2034266 (for 31), and 2034267 (for 12) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.