Boosting Zinc Electrode Reversibility in Aqueous Electrolytes by Using Low-Cost Antisolvents
Dr. Junnan Hao
School of Chemical Engineering & Advanced Materials, The University of Adelaide, Adelaide, SA, 5005 Australia
These authors contributed equally to this work.
Search for more papers by this authorLibei Yuan
Institute for Superconducting and Electronic Materials, Australian Institute for Innovative Materials, University of Wollongong, Wollongong, NSW, 2522 Australia
These authors contributed equally to this work.
Search for more papers by this authorDr. Chao Ye
School of Chemical Engineering & Advanced Materials, The University of Adelaide, Adelaide, SA, 5005 Australia
Search for more papers by this authorDr. Dongliang Chao
School of Chemical Engineering & Advanced Materials, The University of Adelaide, Adelaide, SA, 5005 Australia
Search for more papers by this authorDr. Kenneth Davey
School of Chemical Engineering & Advanced Materials, The University of Adelaide, Adelaide, SA, 5005 Australia
Search for more papers by this authorCorresponding Author
Prof. Zaiping Guo
Institute for Superconducting and Electronic Materials, Australian Institute for Innovative Materials, University of Wollongong, Wollongong, NSW, 2522 Australia
Search for more papers by this authorCorresponding Author
Prof. Shi-Zhang Qiao
School of Chemical Engineering & Advanced Materials, The University of Adelaide, Adelaide, SA, 5005 Australia
Search for more papers by this authorDr. Junnan Hao
School of Chemical Engineering & Advanced Materials, The University of Adelaide, Adelaide, SA, 5005 Australia
These authors contributed equally to this work.
Search for more papers by this authorLibei Yuan
Institute for Superconducting and Electronic Materials, Australian Institute for Innovative Materials, University of Wollongong, Wollongong, NSW, 2522 Australia
These authors contributed equally to this work.
Search for more papers by this authorDr. Chao Ye
School of Chemical Engineering & Advanced Materials, The University of Adelaide, Adelaide, SA, 5005 Australia
Search for more papers by this authorDr. Dongliang Chao
School of Chemical Engineering & Advanced Materials, The University of Adelaide, Adelaide, SA, 5005 Australia
Search for more papers by this authorDr. Kenneth Davey
School of Chemical Engineering & Advanced Materials, The University of Adelaide, Adelaide, SA, 5005 Australia
Search for more papers by this authorCorresponding Author
Prof. Zaiping Guo
Institute for Superconducting and Electronic Materials, Australian Institute for Innovative Materials, University of Wollongong, Wollongong, NSW, 2522 Australia
Search for more papers by this authorCorresponding Author
Prof. Shi-Zhang Qiao
School of Chemical Engineering & Advanced Materials, The University of Adelaide, Adelaide, SA, 5005 Australia
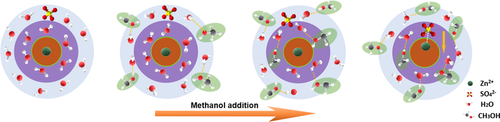
Search for more papers by this authorGraphical Abstract
Water activity and Zn2+ solvation in an ZnSO4 electrolyte are regulated by adding methanol as antisolvent. Methanol gradually interacts with the free and coordinated water in the Zn2+ solvation sheath in the electrolyte, to suppress side reactions and enhance the Zn2+ transference number. Concomitantly, Zn2+ deposition orientation is changed, resulting in dendrite-free Zn deposition and boosted Zn reversibility.
Abstract
Antisolvent addition has been widely studied in crystallization in the pharmaceutical industries by breaking the solvation balance of the original solution. Here we report a similar antisolvent strategy to boost Zn reversibility via regulation of the electrolyte on a molecular level. By adding for example methanol into ZnSO4 electrolyte, the free water and coordinated water in Zn2+ solvation sheath gradually interact with the antisolvent, which minimizes water activity and weakens Zn2+ solvation. Concomitantly, dendrite-free Zn deposition occurs via change in the deposition orientation, as evidenced by in situ optical microscopy. Zn reversibility is significantly boosted in antisolvent electrolyte of 50 % methanol by volume (Anti-M-50 %) even under harsh environments of −20 °C and 60 °C. Additionally, the suppressed side reactions and dendrite-free Zn plating/stripping in Anti-M-50 % electrolyte significantly enhance performance of Zn/polyaniline coin and pouch cells. We demonstrate this low-cost strategy can be readily generalized to other solvents, indicating its practical universality. Results will be of immediate interest and benefit to a range of researchers in electrochemistry and energy storage.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202016531-sup-0001-misc_information.pdf1.5 MB | Supplementary |
| anie202016531-sup-0001-Video_S1.avi36 MB | Supplementary |
| anie202016531-sup-0001-Video_S2.avi26.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aH. Pan, Y. Shao, P. Yan, Y. Cheng, K. S. Han, Z. Nie, C. Wang, J. Yang, X. Li, P. Bhattacharya, Nat. Energy 2016, 1, 16039;
- 1bF. Wan, Z. Niu, Angew. Chem. Int. Ed. 2019, 58, 16358–16367; Angew. Chem. 2019, 131, 16508–16517;
- 1cD. Chao, W. Zhou, F. Xie, C. Ye, H. Li, M. Jaroniec, S.-Z. Qiao, Sci. Adv. 2020, 6, eaba4098;
- 1dC. Zhong, B. Liu, J. Ding, X. Liu, Y. Zhong, Y. Li, C. Sun, X. Han, Y. Deng, N. Zhao, W. Hu, Nat. Energy 2020, 5, 440–449.
- 2
- 2aB. Tang, L. Shan, S. Liang, J. Zhou, Energy Environ. Sci. 2019, 12, 3288–3304;
- 2bM. Song, H. Tan, D. Chao, H. J. Fan, Adv. Funct. Mater. 2018, 28, 1802564;
- 2cX. Zeng, J. Hao, Z. Wang, J. Mao, Z. Guo, Energy Storage Mater. 2019, 20, 410–437;
- 2dZ. Liu, Y. Huang, Y. Huang, Q. Yang, X. Li, Z. Huang, C. Zhi, Chem. Soc. Rev. 2020, 49, 180–232;
- 2eL. E. Blanc, D. Kundu, L. F. Nazar, Joule 2020, 4, 771–799.
- 3
- 3aQ. Zhang, J. Luan, Y. Tang, X. Ji, H. Wang, Angew. Chem. Int. Ed. 2020, 59, 13180–13191; Angew. Chem. 2020, 132, 13280–13291;
- 3bL. Ma, M. A. Schroeder, O. Borodin, T. P. Pollard, M. S. Ding, C. Wang, K. Xu, Nat. Energy 2020, 5, 743–749;
- 3cQ. Yang, G. Liang, Y. Guo, Z. Liu, B. Yan, D. Wang, Z. Huang, X. Li, J. Fan, C. Zhi, Adv. Mater. 2019, 31, 1903778.
- 4
- 4aJ. Hao, X. Li, X. Zeng, D. Li, J. Mao, Z. Guo, Energy Environ. Sci. 2020, 13, 3917–3949;
- 4bL. Ma, S. Chen, N. Li, Z. Liu, Z. Tang, J. A. Zapien, S. Chen, J. Fan, C. Zhi, Adv. Mater. 2020, 32, 1908121.
- 5J. Hao, X. Li, S. Zhang, F. Yang, X. Zeng, S. Zhang, G. Bo, C. Wang, Z. Guo, Adv. Funct. Mater. 2020, 30, 2001263.
- 6
- 6aF. Wang, O. Borodin, T. Gao, X. Fan, W. Sun, F. Han, A. Faraone, J. A. Dura, K. Xu, C. Wang, Nat. Mater. 2018, 17, 543–549;
- 6bW. Yang, X. Du, J. Zhao, Z. Chen, J. Li, J. Xie, Y. Zhang, Z. Cui, Q. Kong, Z. Zhao, Joule 2020, 4, 1557–1574.
- 7
- 7aZ. Zhao, J. Zhao, Z. Hu, J. Li, J. Li, Y. Zhang, C. Wang, G. Cui, Energy Environ. Sci. 2019, 12, 1938–1949;
- 7bJ. Hao, B. Li, X. Li, X. Zeng, S. Zhang, F. Yang, S. Liu, D. Li, C. Wu, Z. Guo, Adv. Mater. 2020, 32, 2003021;
- 7cH. Qiu, X. Du, J. Zhao, Y. Wang, J. Ju, Z. Chen, Z. Hu, D. Yan, X. Zhou, G. Cui, Nat. Commun. 2019, 10, 1–12;
- 7dL. Cao, D. Li, T. Deng, Q. Li, C. Wang, Angew. Chem. Int. Ed. 2020, 59, 19292–19296; Angew. Chem. 2020, 132, 19454–19458.
- 8
- 8aA. Bayaguud, X. Luo, Y. Fu, C. Zhu, ACS Energy Lett. 2020, 5, 3012–3020;
- 8bJ. Hao, J. Long, B. Li, X. Li, S. Zhang, F. Yang, X. Zeng, Z. Yang, W. K. Pang, Z. Guo, Adv. Funct. Mater. 2019, 29, 1903605;
- 8cY. Jin, K. S. Han, Y. Shao, M. L. Sushko, J. Xiao, H. Pan, J. Liu, Adv. Funct. Mater. 2020, 30, 2003932;
- 8dW. Xu, K. Zhao, W. Huo, Y. Wang, G. Yao, X. Gu, H. Cheng, L. Mai, C. Hu, X. Wang, Nano Energy 2019, 62, 275–281.
- 9
- 9aQ. Zhang, J. Luan, L. Fu, S. Wu, Y. Tang, X. Ji, H. Wang, Angew. Chem. Int. Ed. 2019, 58, 15841–15847; Angew. Chem. 2019, 131, 15988–15994;
- 9bS. B. Wang, Q. Ran, R. Q. Yao, H. Shi, Z. Wen, M. Zhao, X. Y. Lang, Q. Jiang, Nat. Commun. 2020, 11, 1634.
- 10
- 10aF. Mo, G. Liang, Q. Meng, Z. Liu, H. Li, J. Fan, C. Zhi, Energy Environ. Sci. 2019, 12, 706–715;
- 10bJ. Zhu, M. Yao, S. Huang, J. Tian, Z. Niu, Angew. Chem. Int. Ed. 2020, 59, 16480–16484; Angew. Chem. 2020, 132, 16622–16626;
- 10cS. Huang, F. Wan, S. Bi, J. Zhu, Z. Niu, J. Chen, Angew. Chem. Int. Ed. 2019, 58, 4313–4317; Angew. Chem. 2019, 131, 4357–4361.
- 11
- 11aJ. Zheng, Q. Zhao, T. Tang, J. Yin, C. D. Quilty, G. D. Renderos, X. Liu, Y. Deng, L. Wang, D. C. Bock, Science 2019, 366, 645–648;
- 11bS. Higashi, S. W. Lee, J. S. Lee, K. Takechi, Y. Cui, Nat. Commun. 2016, 7, 11801;
- 11cJ. Lee, R. Kim, S. Kim, J. Heo, H. Kwon, J. Yang, H. Kim, Energy Environ. Sci. 2020, 13, 2839–2848.
- 12
- 12aL. Zhang, I. A. Pérez, H. Jiang, C. Zhang, D. Leonard, Q. Guo, W. Wang, S. Han, L. Wang, X. Ji, Adv. Funct. Mater. 2019, 29, 1902653;
- 12bH. Yang, Z. Chang, Y. Qiao, H. Deng, X. Mu, P. He, H. Zhou, Angew. Chem. Int. Ed. 2020, 59, 9377–9381; Angew. Chem. 2020, 132, 9463–9467.
- 13L. Suo, O. Borodin, T. Gao, M. Olguin, J. Ho, X. Fan, C. Luo, C. Wang, K. Xu, Science 2015, 350, 938–943.
- 14J. Xie, Z. Liang, Y. Lu, Nat. Mater. 2020, 19, 1006–1011.
- 15
- 15aA. A. Thorat, S. V. Dalvi, Chem. Eng. J. 2012, 181, 1–34;
- 15bY. Y. Kim, T. Yang, R. Suhonen, A. Kemppainen, K. Hwang, N. J. Jeon, J. Seo, Nat. Commun. 2020, 11, 3581;
- 15cW. Xu, Y. Gao, W. Ming, F. He, J. Li, X. H. Zhu, F. Kang, J. Li, G. Wei, Adv. Mater. 2020, 32, 2003965.
- 16Y. Yun, F. Wang, H. Huang, Y. Fang, S. Liu, W. Huang, Z. Cheng, Y. Liu, Y. Cao, M. Gao, Adv. Mater. 2020, 32, 1907123.
- 17T. Zhang, F. Wang, H. Chen, L. Ji, Y. Wang, C. Li, M. B. Raschke, S. Li, ACS Energy Lett. 2020, 5, 1619–1627.
- 18
- 18aH. Liu, K. L. Sale, B. A. Simmons, S. Singh, J. Phys. Chem. B 2011, 115, 10251–10258;
- 18bT. C. Schutt, G. A. Hegde, V. S. Bharadwaj, A. J. Johns, C. M. Maupin, J. Phys. Chem. B 2017, 121, 843–853.
- 19A. T. Aguayo, A. G. Gayubo, R. Vivanco, M. Olazar, J. Bilbao, Appl. Catal. A 2005, 283, 197–207.
- 20M. Mohsen-Nia, H. Amiri, B. Jazi, J. Solution Chem. 2010, 39, 701–708.
- 21H. Gottlieb, V. Kotlyar, A. Nudelman, J. Org. Chem. 1997, 62, 7512–7515.
- 22Y. Yu, W. Fan, Y. Wang, X. Zhou, J. Sun, S. Liu, J. Phys. Chem. B 2017, 121, 8179–8187.
- 23M. I. Sulaiman, S. Yang, A. M. Ellis, J. Phys. Chem. A 2017, 121, 771–776.
- 24W. W. Rudolph, M. H. Brooker, P. Tremaine, Z. Phys. Chem. 1999, 209, 181–207.
- 25J. Ming, Z. Cao, W. Wahyudi, M. Li, P. Kumar, Y. Wu, J. Hwang, M. N. Hedhili, L. Cavallo, Y. Sun, ACS Energy Lett. 2018, 3, 335–340.
- 26Q. Zhang, Y. Ma, Y. Lu, L. Li, F. Wan, K. Zhang, J. Chen, Nat. Commun. 2020, 11, 4463.
- 27N. Chang, T. Li, R. Li, S. Wang, Y. Yin, H. Zhang, X. Li, Energy Environ. Sci. 2020, 13, 3527–3535.
- 28
- 28aX. Zeng, J. Liu, J. Mao, J. Hao, Z. Wang, S. Zhou, C. D. Ling, Z. Guo, Adv. Energy Mater. 2020, 10, 1904163;
- 28bD. Chao, W. Zhou, C. Ye, Q. Zhang, Y. Chen, L. Gu, K. Davey, S.-Z. Qiao, Angew. Chem. Int. Ed. 2019, 58, 7823–7828; Angew. Chem. 2019, 131, 7905–7910.
- 29D. Chao, S.-Z. Qiao, Joule 2020, 4, 1846–1851.
- 30C. Li, Z. Sun, T. Yang, L. Yu, N. Wei, Z. Tian, J. Cai, J. Lv, Y. Shao, M. H. Rümmeli, Adv. Mater. 2020, 32, 2003425.
- 31Q. Guan, Y. Li, X. Bi, J. Yang, J. Zhou, X. Li, J. Cheng, Z. Wang, B. Wang, J. Lu, Adv. Energy Mater. 2019, 9, 1901434.
- 32T. Zhang, Y. Tang, G. Fang, C. Zhang, H. Zhang, X. Guo, X. Cao, J. Zhou, A. Pan, S. Liang, Adv. Funct. Mater. 2020, 30, 2002711.
- 33Y. Suleymanov, Science 2019, 366, 321–322.
- 34J. Wang, W. Huang, A. Pei, Y. Li, F. Shi, X. Yu, Y. Cui, Nat. Energy 2019, 4, 664–670.
- 35
- 35aQ. Zhang, J. Luan, X. Huang, Q. Wang, D. Sun, Y. Tang, X. Ji, H. Wang, Nat. Commun. 2020, 11, 3961;
- 35bK. E. Sun, T. K. Hoang, T. N. L. Doan, Y. Yu, X. Zhu, Y. Tian, P. Chen, ACS Appl. Mater. Interfaces 2017, 9, 9681–9687.
- 36F. Wan, L. Zhang, X. Wang, S. Bi, Z. Niu, J. Chen, Adv. Funct. Mater. 2018, 28, 1804975.