Synthetic C6-Functionalized Aminoflavin Catalysts Enable Aerobic Bromination of Oxidation-Prone Substrates
Graphical Abstract
Upon photoexcitation with visible light, synthetic C6 aminoflavin catalysts are reversibly reduced by 2,6-lutidinium oxalate and allow the activation of molecular oxygen. In the presence of halide salt, the strategy is successful in the biomimetic halogenation of oxidation-prone tyrosine, flavone, as well as flavanone substrates, while natural (−)-riboflavin leads to decomposition.
Abstract
Flavoenzymes catalyze oxidations via hydroperoxide intermediates that result from activation of molecular O2. These reactions—such as hydroxylation and halogenation—depend on the additional catalytic activity of functional groups in the peptide environment of the flavin cofactor. We report synthetic flavin catalysts that contain C6 amino modifications at the isoalloxazine core and are consequently capable of mediating halogenations outside the peptide surrounding. The catalysts are competent in the selective, biomimetic bromination of oxidation-prone phenols, flavones, and flavanones using a halide salt in combination with 2,6-lutidinium oxalate as a flavin reductant under visible-light irradiation. Our studies show the beneficial effect of stacked bisflavins as well as the catalytic activity of the flavin modifications. The designed flavin catalysts outperform isolated natural (−)-riboflavin and contribute to the continuing search for tailored flavins in oxidation reactions.
In nature, flavoenzymes mediate a large variety of chemical transformations relying on the identical cofactor flavin adenine dinucleotide (FAD).1 Upon conversion to FADH2 by reduced nicotinamide adenine dinucleotide (NADH), they are able to activate molecular oxygen from air, which occurs via one electron reduction and HOO. radical recombination with the cofactor (Figure 1 A, left side).2 The resulting C4a hydroperoxide is a highly activated reactive intermediate—with more than 103-fold increased reactivity for oxygen transfer when compared to hydrogen peroxide3—and mediates reactions including olefin epoxidation,4 hydroxylation,5 heteroatom oxidation,6 and Baeyer–Villiger oxidation.7 A unique subset of these oxidations is the conversion of inorganic halide salts into hypohalites in halogenase enzymes. Mechanistic studies suggest that hypohalites are transferred to substrates via a lysine residue's primary amino group, which either serves as hydrogen bond donor when protonated or is converted to an intermediate haloamine (Figure 1 A, right side).8
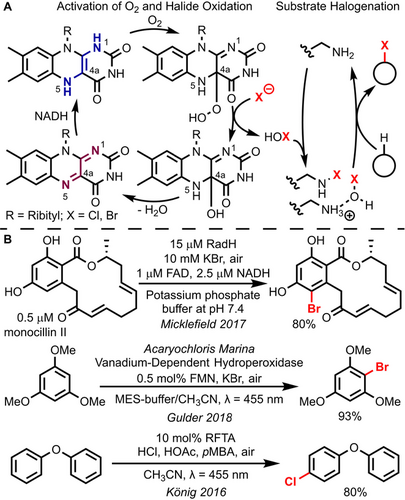
Aerobic halogenation reactions with flavins. A) Mechanistic proposal for flavoenzymatic halogenation. B) Representative strategies for flavin-mediated halogenations in organic synthesis. RFTA: (−)-Riboflavin tetraacetate.
Flavoenzymatic halogenations have also been transferred to the organic laboratory,9 highlighted by the successful application of the fungal halogenase RadH in mono-bromination of monocillin II and other bioactive compounds (Figure 1 B, top).10 While in general the potential of flavin-mediated transformations for organic synthesis has been recognized,11, 12 reports on biomimetic flavin-catalyzed halogenation are scarce—presumably due to the absence of catalytically active residues, when the cofactor is isolated from the enzymatic surrounding. One strategy was reported by the Gulder laboratory and centered around the flavin mononucleotide (FMN) cofactor, which was used for conversion of O2 from air to hydrogen peroxide in combination with vanadium-dependent enzymes for halogenation of aromatic substrates (Figure 1 B, middle).13 The first fully non-enzymatic flavin-mediated halogenation reaction was reported by König et al. using (−)-riboflavin for hydrogen peroxide generation and subsequent oxidation of hydrochloric acid (Figure 1 B, bottom).14 The authors used para-methoxybenzyl alcohol (pMBA) as reductant for photoexcited (−)-riboflavin and identified acetic acid as crucial catalytic shuttle for hydrogen peroxide via intermediate peracetic acid formation under the acidic conditions.
We were intrigued by the idea to synthetically modify non-enzymatic flavin catalysts in order to achieve activation of molecular oxygen as well as electrophilic halogenation without the need of additional catalysts or mediators. Moreover, we aimed to facilitate these reactions in organic solvents and also to apply them successfully to oxidation-prone substances containing free phenols, which would significantly increase the synthetic utility of these reactions. Modification of the isoalloxazine core with an amino group seemed promising, since the resulting flavins were anticipated to be less oxidizing,15 reducing substrate decomposition, while also providing a synthetic handle for installing hydrogen bond donors or nitrogen-based Lewis bases in analogy to the lysine side chain activity. Two synthetic flavin designs were studied (Figure 2 A): First, in flavins based on amine 1, the C6 position is functionalized in order to position the “side arm” close to the reactive quinoid center. Second, bisflavins 2, based on trans-1,2-diaminocyclohexane,16 contain two isoalloxazines in a stacked arrangement, thereby locating the C6 functionality proximal to the opposite side's quinoid center as well.
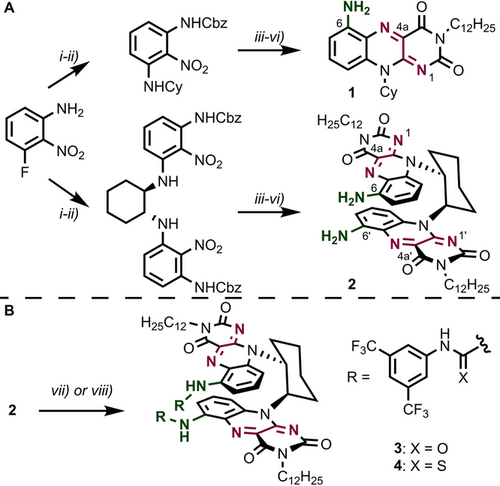
Synthesis of C6 aminoflavin catalysts. A) Route to aminoflavin platform compounds. i) CbzCl, MgO, 71 %. Preparation of 1: ii) CyNH2, K2CO3, 93 %; iii) SnCl2, quant.; iv) alloxan, B2O3, 92 %; v) C12H25I, K2CO3, 65 %; vi) HBr, 77 %. Preparation of 2: ii) (R,R)-DACH, K2CO3, 78 %; iii) SnCl2, 85 %; iv) alloxan, B2O3, 78 %; v) C12H25I, K2CO3, 94 %; vi) HBr, 86 %. B) Functionalization of 2: vii) 3,5-bis(trifluoromethyl)phenyl isocyanate, 48 %, viii) 3,5-bis(trifluoromethyl)phenyl isothiocyanate, 65 %. Functionalizations of 1 were performed analogously. DACH: 1,2-diaminocyclohexane.
Both synthetic procedures commence with 3-fluoro-2-nitroaniline and consist of a sequence of Cbz protection, SNAr reaction, nitro group reduction, condensation with alloxan, N3 alkylation, and Cbz deprotection (Figure 2 A). Amines 1 and 2—bench stable, deep black compounds—were obtained in multigram quantities and N3 dodecylation ensures their solubility in a wide range of organic solvents. The C6 amino groups were converted into urea (3) and thiourea (4) functionalities, which serve as efficient hydrogen bond donors (Figure 2 B).
With our synthetic route established, we then focused on the reduction of the isoalloxazine core as the first crucial step in aerobic flavin halogenation (see Figure 1 A). A number of suitable reductants is known,11 including Hantzsch ester, dithionite, zinc, and hydrazine, however, none of the common reductants is (i) soluble in organic solvents, (ii) unreactive towards other functional groups, and additionally (iii) not quenched by oxidizing catalysis intermediates such as hydroperoxides, hypohalous acids, or hydrogen peroxide. Inspired by studies on oxalate oxidase enzymes and the activity of photoexcited (−)-riboflavin in CO2 release from oxalic acid,17 we became interested in using the latter as convenient flavin reductant. Oxalic acid itself is insoluble in organic solvent but 2,6-lutidinium oxalate (2:1 mixture, “LutOx”) indeed acts as a competent reductant for (−)-riboflavin tetraacetate (RFTA) in dichloromethane upon irradiation with blue LED light (Figure 3 A and supporting information).
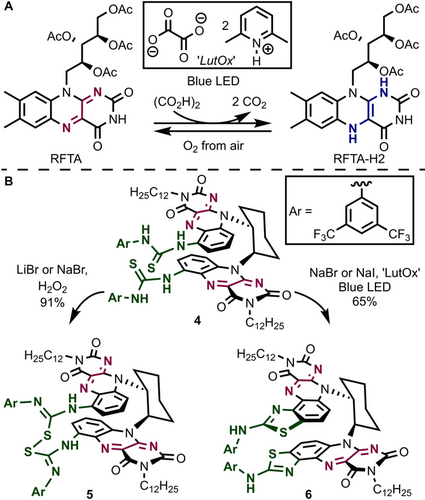
Reversible flavin reduction using 2,6-lutidinium oxalate (“LutOx”). A) Reduction of RFTA in dichloromethane. B) Divergent reactivity of thiourea flavin 4 upon exposure to aerobic conditions and halide salt.
We then studied the reactivity of thiourea flavin 4 towards hydrogen peroxide—as a mimic for flavin C4a hydroperoxides—in the presence of inorganic halide salt (Figure 3 B). Within minutes, we observed selective formation (91 % isolated yield) of disulfide-type compound 5 by a reaction that proceeds especially well in dilute solution (1.5 mm, see supporting information) and is similar to the dimerization of simple alkyl thioureas.18 In stark contrast, when irradiated with “LutOx” under otherwise similar conditions, clean Jacobsen-Hugershoff-type19 reaction occurred and bis-2-aminobenzothiazole flavin 6 was formed. This divergence is also observed when 4 is treated with excess I2 under argon, which leads to 5 in the dark while 6 is formed predominantly under blue LED irradiation. We assume that a photoredox mechanism is operative under irradiation and outcompetes sulfur-sulfur bond formation. Consistent with this analysis, the thiourea derived from monoflavin 1 shows 2-aminobenzothiazole (7) formation within minutes also when using H2O2 and LiBr since no competing intramolecular reaction partner is present.
We then studied, whether benzothiazole flavins 6 and 7 are also reduced by “LutOx” via irradiation under inert conditions (Figure 4). They appear as deep red compounds with absorption in the visible range (λmax=420 (6) and 426 nm (7) in CH2Cl2) and show fluorescence (λex=410 nm, see supporting information) at λmax=593 (6), and 573 nm (7). This prompted us to apply blue LED light for excitation and indeed, clean reduction of 7 occurred. When exposed to air, the sample was re-oxidized concomitant to O2 reduction. This method was also suitable for bisflavin 6 albeit significantly slower when compared to 7. Urea flavins 3 and 8 (urea derived from 1) were reduced even more slowly (see supporting information). Decomposition was observed when applying the conditions to disulfide 5.
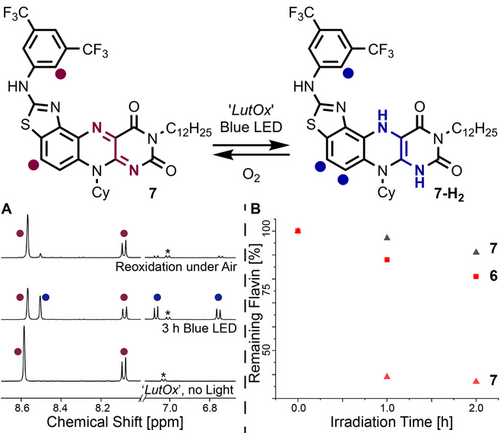
“LutOx” reduction of benzothiazole flavin catalysts. A) Under argon, a sample of 7 and “LutOx” (5 equiv) in CD2Cl2 was irradiated for 3 h with a blue Kessil® LED (43 % reduction). The tube was then opened to air. B) Photochemical “LutOx” reductions of 6 and 7 under O2 with (red) and without (black) lithium bromide. Concentrations were monitored by HPLC.
Before employing the flavin catalysts in aerobic halogenation reactions with inorganic halide sources, we probed the stability of catalysts 6 and 7 under these conditions. When irradiated (λmax=457 nm, LED) under O2 (Figure 4 B, black triangles) in the presence of “LutOx” reductant, 7 was observed to be almost fully intact (>90 %) after 2 h. However, when inorganic lithium bromide salt was added, rapid decomposition occurred (red triangles). The catalyst lifetime of 6 was much higher (red squares), presumably as a result of the continuous slow generation of Br+ equivalents. This encouraged us to apply flavin 6 in catalysis.
As a proof-of-principle reaction, we chose the biomimetic halogenation of model tyrosine 9 with molecular O2 and inorganic bromide salt in dichloromethane as solvent (Figure 5). These conditions were expected to be compatible with the oxidation-prone phenol as well as acid- (N-Boc) and base-sensitive (methyl ester) protecting groups. Parent (−)-riboflavin tetraacetate (RFTA) resulted in unselective substrate decomposition, and only 12 % bromination products were observed (Entry 1). Shorter reaction times did not result in increased yield. We then moved to C6 amino monoflavins (entries 2–3) and observed that urea catalyst 8 led to less decomposition of 9, but both reactions did not improve the bromination yield. However, we noted that the experiment with monoflavin 7 had decolored during the course of the reaction, and after applying shorter reaction times, the yield of bromination increased. We aimed for beneficial effects of slow and controlled formation of oxidizing species using bisflavins 3, 5, and 6 under oxidative halogenation conditions, and indeed decreased decomposition was observed in these cases (entries 4–6). While 3 and 5 did not improve the product yield, benzothiazole 6 resulted in 58 % bromination with a good overall mass balance of 70 %. Control experiments confirmed that light, flavin catalyst, and reductant are each required (see supporting information, which also contains a solvent screening). The optimal catalyst loading was 5 mol %, which highlights the flavin stability.
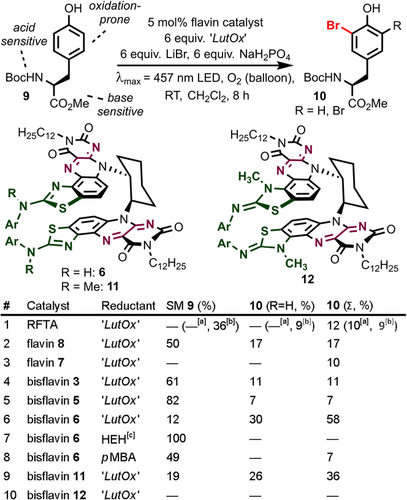
Application of C6 aminoflavin catalysts in the aerobic, biomimetic halogenation of model tyrosine 9. Sodium dihydrogen phosphate was added as a mild proton source. Yields were determined by NMR spectroscopy versus internal standard. [a] Reaction time 4 h. [b] Reaction time 45 min. [c] No irradiation since Hantzsch ester (HEH) reduces 6 in the dark. Ar=3,5-Bis(trifluoromethyl)phenyl. SM: Starting material.
Hantzsch ester20 and para-methoxybenzyl alcohol21—both competent flavin reductants—did not yield significant quantities of 10 under our conditions (entries 7–8). Interestingly, the N-methylated catalyst 11 showed diminished activity, and positional isomer 12 did not even yield 10 in any measurable quantity (entries 9–10). This observation stimulated our interest in probing the catalytic activity of the 2-aminobenzothiazole functionality during halogenation catalysis. Therefore, we studied the activity of flavin 7 in two different routes for bromination of tyrosine derivative 9, which mimic the early and late stage processes of the full biomimetic halogenation sequence. We first probed for activity when using tert-butylhydroperoxide—as flavin C4a hydroperoxide mimic—in combination with lithium bromide under otherwise identical catalytic conditions (see supporting information). Indeed, flavin 7 showed significant catalytic activity. In a second approach, we used N-bromosuccinimide as Br+-source and again found rate enhancement when using flavin 7 as catalyst (see supporting information). Both experiments point towards active catalytic participation of the 2-aminobenzothiazole group in bromination catalysis.
Having observed bromination over unselective substrate decomposition, we expanded our catalysis studies to different kinds of oxidation-prone, phenolic substrates (Figure 6). Other N-terminal tyrosine protecting groups such as Cbz (13) and Fmoc (14) are tolerated, which highlights the compatibility with oxidation-prone benzylic positions. Dipeptides like Boc-Tyr-Val-OMe 15 are suitable substrates as well. Additionally, a variety of flavones, umbelliferon, and flavanones resulted in efficient, regioselective mono-bromination (16–19). Throughout all examples, (−)-riboflavin tetraacetate led to decomposition independent of the chosen reaction time (see supporting information).
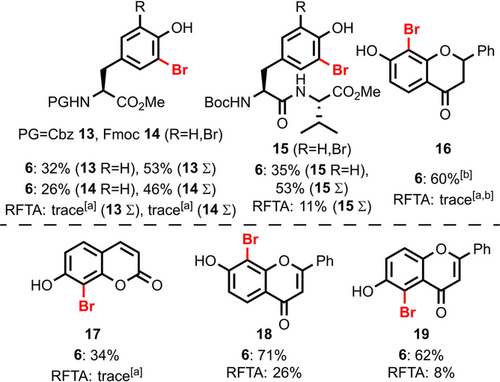
Application of bisflavin 6 in aerobic halogenation of oxidation-prone substrates. Yields were determined by NMR spectroscopy versus internal standard. See Figure 5 for reaction conditions, with 12 equiv “LutOx” used for 19 and 15 equiv “LutOx” for 16–18. [a] A yield of ≤5 % was observed. [b] Additionally, 29 % (with 6) and 21 % (with RFTA) of dibromination observed. PG: Protecting group.
In summary, we report the design and characterization of a series of C6 aminoflavin catalysts and their reactivity under aerobic conditions. We identified 2,6-lutidinium oxalate as organic soluble, inexpensive, and traceless reductant for photoexcited flavins. Applying this reduction strategy, we observed selective transformations of thiourea flavins, resulting in 2-aminobenzothiazole functionalities. Under aerobic conditions, stacked bisflavins were found to be very stable catalysts, which slowly release oxidants upon activation of O2. Building on these studies, we developed a flavin-mediated halogenation strategy for oxidation-prone substrates including phenolic compounds, flavones, and flavanones. One key advantage when compared to parent (−)-riboflavin is the significantly suppressed substrate decomposition. Mechanistic studies additionally revealed catalytic participation of the 2-aminobenzothiazole modification. The present study is, therefore, anticipated to serve as critical starting point for site-selective flavin catalysis.
Acknowledgements
The Chemical Industry Funds (PhD Fellowship to A.W. and Liebig Fellowship to G.S.) is gratefully acknowledged. Our group is supported by the Technical University of Munich through the Junior Fellow Programme. G.S. is very grateful to Prof. T. Bach for his continuous support. We thank Dr. A. Bauer for assistance with UV/Vis spectroscopy and O. Ackermann for HPLC analyses. Open access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.