Tetra-benzothiadiazole-based [12]Cycloparaphenylene with Bright Emission and Its Supramolecular Assembly
Zhen-Lin Qiu
State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorChun Tang
State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorXin-Rong Wang
State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorYang-Yang Ju
State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorKe-Shan Chu
State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorZe-Ying Deng
State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorHao Hou
State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorDr. Yu-Min Liu
State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Yuan-Zhi Tan
State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorZhen-Lin Qiu
State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorChun Tang
State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorXin-Rong Wang
State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorYang-Yang Ju
State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorKe-Shan Chu
State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorZe-Ying Deng
State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorHao Hou
State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorDr. Yu-Min Liu
State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Yuan-Zhi Tan
State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorGraphical Abstract
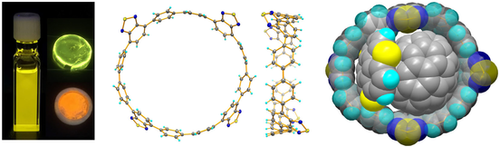
A benzothiadiazole-based [12]cycloparaphenylene (TB[12]CPP) is obtained and characterized. TB[12]CPP exhibits a lime to orange emission with a quantum yield up to 98 %, ranking it as one of the brightest CPPs. As a supramolecular host, TB[12]CPP is bright as well, showing adaptable ring structure. A ternary assembly between TB[12]CPP, fullerene, and buckybowl is realized.
Abstract
The radial conjugated π-system of cycloparaphenylenes (CPPs) makes them intriguing fluorophores and unique supramolecular hosts. However, the bright photoluminescence (PL) of CPPs was limited to the blue light and the supramolecular assembly behavior of large CPPs was rarely investigated. Here we present the synthesis of tetra-benzothiadiazole-based [12]cycloparaphenylene (TB[12]CPP), which exhibits a lime to orange PL with an excellent quantum yield up to 82 % in solution. The PL quantum yield of TB[12]CPP can be further improved to 98 % in polymer matrix. Benefiting from its enlarged size, TB[12]CPP can accommodate a fullerene derivative or concave–convex complexes of fullerene and buckybowl through the combined π–π and C−H⋅⋅⋅π interactions. The latter demonstrates the first case of a ternary supramolecule of CPPs.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202008505-sup-0001-misc_information.pdf1.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. E. Lewis, Chem. Soc. Rev. 2015, 44, 2221–2304.
- 2H. Omachi, T. Nakayama, E. Takahashi, Y. Segawa, K. Itami, Nat. Chem. 2013, 5, 572–576.
- 3
- 3aW. S. Rapson, R. G. Shuttleworth, J. N. van Niekerk, J. Chem. Soc. 1943, 326–327;
- 3bH. A. Staab, F. Binnig, Tetrahedron Lett. 1964, 5, 319–321.
10.1016/0040-4039(64)80020-4 Google Scholar
- 4
- 4aR. Jasti, J. Bhattacharjee, J. B. Neaton, C. R. Bertozzi, J. Am. Chem. Soc. 2008, 130, 17646–17647;
- 4bH. Takaba, H. Omachi, Y. Yamamoto, J. Bouffard, K. Itami, Angew. Chem. Int. Ed. 2009, 48, 6112–6116; Angew. Chem. 2009, 121, 6228–6232.
- 5E. J. Leonhardt, R. Jasti, Nat. Rev. Chem. 2019, 3, 672–686.
- 6E. R. Darzi, R. Jasti, Chem. Soc. Rev. 2015, 44, 6401–6410.
- 7
- 7aH. Tang, Z. Gu, C. Li, Z. Li, W. Wu, X. Jiang, Biomater. Sci. 2019, 7, 2552–2558;
- 7bB. M. White, Y. Zhao, T. E. Kawashima, B. P. Branchaud, M. D. Pluth, R. Jasti, ACS Cent. Sci. 2018, 4, 1173–1178.
- 8
- 8aS. Yamago, Y. Watanabe, T. Iwamoto, Angew. Chem. Int. Ed. 2010, 49, 757–759; Angew. Chem. 2010, 122, 769–771;
- 8bT. J. Sisto, M. R. Golder, E. S. Hirst, R. Jasti, J. Am. Chem. Soc. 2011, 133, 15800–15802.
- 9
- 9aE. R. Darzi, E. S. Hirst, C. D. Weber, L. N. Zakharov, M. C. Lonergan, R. Jasti, ACS Cent. Sci. 2015, 1, 335–342;
- 9bT. Kuwabara, J. Orii, Y. Segawa, K. Itami, Angew. Chem. Int. Ed. 2015, 54, 9646–9649; Angew. Chem. 2015, 127, 9782–9785.
- 10
- 10aY. Xu, M. von Delius, Angew. Chem. Int. Ed. 2020, 59, 559–573; Angew. Chem. 2020, 132, 567–582;
- 10bD. Lu, Q. Huang, S. Wang, J. Wang, P. Huang, P. Du, Front. Chem. 2019, 7, 668;
- 10cM. B. Minameyer, Y. Xu, S. Frühwald, A. Görling, M. von Delius, T. Drewello, Chem. Eur. J. 2020, https://doi.org/10.1002/chem.202001503.
- 11
- 11aT. Iwamoto, Y. Watanabe, T. Sadahiro, T. Haino, S. Yamago, Angew. Chem. Int. Ed. 2011, 50, 8342–8344; Angew. Chem. 2011, 123, 8492–8494;
- 11bT. Iwamoto, Y. Watanabe, H. Takaya, T. Haino, N. Yasuda, S. Yamago, Chem. Eur. J. 2013, 19, 14061–14068.
- 12
- 12aT. Matsuno, K. Fukunaga, S. Sato, H. Isobe, Angew. Chem. Int. Ed. 2019, 58, 12170–12174; Angew. Chem. 2019, 131, 12298–12302;
- 12bT. Matsuno, M. Fujita, K. Fukunaga, S. Sato, H. Isobe, Nat. Commun. 2018, 9, 3779.
- 13D. Lu, G. Zhuang, H. Wu, S. Wang, S. Yang, P. Du, Angew. Chem. Int. Ed. 2017, 56, 158–162; Angew. Chem. 2017, 129, 164–168.
- 14Y. Wang, T. Michinobu, J. Mater. Chem. C 2016, 4, 6200–6214.
- 15T. C. Lovell, Z. R. Garrison, R. Jasti, Angew. Chem. Int. Ed. 2020, 59, 14363–14367; Angew. Chem. 2020, 132, 14469–14473.
- 16I. Alkorta, I. Rozas, J. Elguero, Chem. Soc. Rev. 1998, 27, 163–170.
- 17Y. Segawa, S. Miyamoto, H. Omachi, S. Matsuura, P. Šenel, T. Sasamori, N. Tokitoh, K. Itami, Angew. Chem. Int. Ed. 2011, 50, 3244–3248; Angew. Chem. 2011, 123, 3302–3306.
- 18E. R. Darzi, T. J. Sisto, R. Jasti, J. Org. Chem. 2012, 77, 6624–6628.
- 19T. C. Lovell, C. E. Colwell, L. N. Zakharov, R. Jasti, Chem. Sci. 2019, 10, 3786–3790.
- 20C. Reichardt, Chem. Rev. 1994, 94, 2319–2358.
- 21M. Nishio, Phys. Chem. Chem. Phys. 2011, 13, 13873–13900.
- 22Q. Tan, D. Zhou, T. Zhang, B. Liu, B. Xu, Chem. Commun. 2017, 53, 10279–10282.
- 23Deposition Numbers 2009760, 2009761, 2009762, and 2018161 contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.