Stereoinduction in Metallaphotoredox Catalysis
Dr. Alexander Lipp
Department of Chemistry, Roy and Diana Vagelos Laboratories, University of Pennsylvania, 231 S. 34th Street, Philadelphia, PA, 19104-6323 USA
These authors contributed equally to this work.
Search for more papers by this authorShorouk O. Badir
Department of Chemistry, Roy and Diana Vagelos Laboratories, University of Pennsylvania, 231 S. 34th Street, Philadelphia, PA, 19104-6323 USA
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Dr. Gary A. Molander
Department of Chemistry, Roy and Diana Vagelos Laboratories, University of Pennsylvania, 231 S. 34th Street, Philadelphia, PA, 19104-6323 USA
Search for more papers by this authorDr. Alexander Lipp
Department of Chemistry, Roy and Diana Vagelos Laboratories, University of Pennsylvania, 231 S. 34th Street, Philadelphia, PA, 19104-6323 USA
These authors contributed equally to this work.
Search for more papers by this authorShorouk O. Badir
Department of Chemistry, Roy and Diana Vagelos Laboratories, University of Pennsylvania, 231 S. 34th Street, Philadelphia, PA, 19104-6323 USA
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Dr. Gary A. Molander
Department of Chemistry, Roy and Diana Vagelos Laboratories, University of Pennsylvania, 231 S. 34th Street, Philadelphia, PA, 19104-6323 USA
Search for more papers by this authorGraphical Abstract
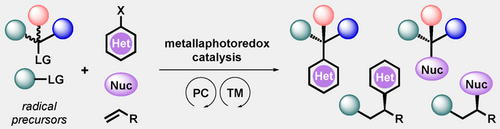
The synergistic combination of photoredox and transition metal catalysis enables the installation of C(sp3)-hybridized centers under exceedingly low energy barriers, rendering this platform a formidable tool in synthetic applications. To fully harness this mode of catalysis, considerable efforts have been devoted toward stereoselective transformations. This Minireview discusses milestones to accomplish and understand the origin of stereoinduction in these processes.
Abstract
Metallaphotoredox catalysis has evolved into an enabling platform to construct C(sp3)-hybridized centers under remarkably mild reaction conditions. The cultivation of abundant radical precursor feedstocks has significantly increased the scope of transition-metal-catalyzed cross-couplings, especially with respect to C(sp2)–C(sp3) linkages. In recent years, considerable effort has been devoted to understanding the origin of stereoinduction in dual catalytic processes. In this context, Ni- and Cu-catalyzed transformations have played a predominant role exploiting this mode of catalysis. Herein, we provide a critical overview on recent progress in enantioselective bond formations enabled by Ni- and Cu-catalyzed manifolds. Furthermore, selected stereochemical control elements within the realm of diastereoselective transformations are discussed.
Conflict of interest
The authors declare no conflict of interest.
References
- 1
- 1aJ. C. Tellis, D. N. Primer, G. A. Molander, Science 2014, 345, 433–436;
- 1bZ. Zuo, D. T. Ahneman, L. Chu, J. A. Terrett, A. G. Doyle, D. W. MacMillan, Science 2014, 345, 437–440;
- 1cD. N. Primer, I. Karakaya, J. C. Tellis, G. A. Molander, J. Am. Chem. Soc. 2015, 137, 2195–2198;
- 1dB. J. Shields, A. G. Doyle, J. Am. Chem. Soc. 2016, 138, 12719–12722. For selected reviews, please see:
- 1eJ. C. Tellis, C. B. Kelly, D. N. Primer, M. Jouffroy, N. R. Patel, G. A. Molander, Acc. Chem. Res. 2016, 49, 1429–1439;
- 1fK. L. Skubi, T. R. Blum, T. P. Yoon, Chem. Rev. 2016, 116, 10035–10074;
- 1gJ. A. Milligan, J. P. Phelan, S. O. Badir, G. A. Molander, Angew. Chem. Int. Ed. 2019, 58, 6152–6163; Angew. Chem. 2019, 131, 6212–6224;
- 1hJ. Twilton, C. Le, P. Zhang, M. H. Shaw, R. W. Evans, D. W. C. MacMillan, Nat. Rev. Chem. 2017, 1, 0052;
- 1iJ. K. Matsui, S. B. Lang, D. R. Heitz, G. A. Molander, ACS Catal. 2017, 7, 2563–2575;
- 1jM. Parasram, V. Gevorgyan, Chem. Soc. Rev. 2017, 46, 6227–6240.
- 2
- 2aF. Lovering, J. Bikker, C. Humblet, J. Med. Chem. 2009, 52, 6752–6756;
- 2bF. Lovering, MedChemComm 2013, 4, 515–519.
- 3For selected examples of complementary radical-based asymmetric Ni- and Cu-catalyzed transformations, please see:
- 3aY. Zhao, D. J. Weix, J. Am. Chem. Soc. 2015, 137, 3237–3240;
- 3bQ.-S. Gu, Z.-L. Li, X.-Y. Liu, Acc. Chem. Res. 2020, 53, 170–181;
- 3cZ.-L. Li, G.-C. Fang, Q.-S. Gu, X.-Y. Liu, Chem. Soc. Rev. 2020, 49, 32–48;
- 3dK. E. Poremba, S. E. Dibrell, S. E. Reisman, ACS Catal. 2020, 10, 8237–8246. For recent reviews on enantioselective reactions in photoinduced systems, please see:
- 3eJ. Busch, D. M. Knoll, C. Zippel, S. Bräse, C. Bizzarri, Dalton Trans. 2019, 48, 15338–15357;
- 3fH.-H. Zhang, H. Chen, C. Zhu, S. Yu, Sci. China Chem. 2020, 63, 637–647.
- 4J. F. Hartwig, Organotransition Metal Chemistry: from Bonding to Catalysis, University Science Books, Sausalito, Calif., 2010.
- 5O. Gutierrez, J. C. Tellis, D. N. Primer, G. A. Molander, M. C. Kozlowski, J. Am. Chem. Soc. 2015, 137, 4896–4899.
- 6D. Ryu, D. N. Primer, J. C. Tellis, G. A. Molander, Chem. Eur. J. 2016, 22, 120–123.
- 7J. Amani, E. Sodagar, G. A. Molander, Org. Lett. 2016, 18, 732–735.
- 8E. E. Stache, T. Rovis, A. G. Doyle, Angew. Chem. Int. Ed. 2017, 56, 3679–3683; Angew. Chem. 2017, 129, 3733–3737.
- 9Q.-Q. Zhou, F.-D. Lu, D. Liu, L.-Q. Lu, W.-J. Xiao, Org. Chem. Front. 2018, 5, 3098–3102.
- 10Z. Zuo, H. Cong, W. Li, J. Choi, G. C. Fu, D. W. MacMillan, J. Am. Chem. Soc. 2016, 138, 1832–1835.
- 11C. Pezzetta, D. Bonifazi, R. W. M. Davidson, Org. Lett. 2019, 21, 8957–8961.
- 12K. Godula, D. Sames, Science 2006, 312, 67–72.
- 13D. T. Ahneman, A. G. Doyle, Chem. Sci. 2016, 7, 7002–7006.
- 14Y. Shen, Y. Gu, R. Martin, J. Am. Chem. Soc. 2018, 140, 12200–12209.
- 15A. W. Rand, H. Yin, L. Xu, J. Giacoboni, R. Martin-Montero, C. Romano, J. Montgomery, R. Martin, ACS Catal. 2020, 10, 4671–4676.
- 16X. Cheng, H. Lu, Z. Lu, Nat. Commun. 2019, 10, 3549.
- 17D. R. Heitz, J. C. Tellis, G. A. Molander, J. Am. Chem. Soc. 2016, 138, 12715–12718.
- 18H. Guan, Q. Zhang, P. J. Walsh, J. Mao, Angew. Chem. Int. Ed. 2020, 59, 5172–5177; Angew. Chem. 2020, 132, 5210–5215.
- 19W. Ding, L. Q. Lu, Q. Q. Zhou, Y. Wei, J. R. Chen, W. J. Xiao, J. Am. Chem. Soc. 2017, 139, 63–66.
- 20E. Gandolfo, X. Tang, S. Raha Roy, P. Melchiorre, Angew. Chem. Int. Ed. 2019, 58, 16854–16858; Angew. Chem. 2019, 131, 17010–17014.
- 21P. Fan, Y. Lan, C. Zhang, C. Wang, J. Am. Chem. Soc. 2020, 142, 2180–2186.
- 22
- 22aA. Hossain, A. Bhattacharyya, O. Reiser, Science 2019, 364, eaav9713;
- 22bM. Zhong, X. Pannecoucke, P. Jubault, T. Poisson, Beilstein J. Org. Chem. 2020, 16, 451–481;
- 22cR. Kancherla, K. Muralirajan, A. Sagadevan, M. Rueping, Trends Chem. 2019, 1, 510–523;
- 22dT. P. Nicholls, A. C. Bissember, Tetrahedron Lett. 2019, 60, 150883;
- 22eO. Reiser, Acc. Chem. Res. 2016, 49, 1990–1996.
- 23Q. M. Kainz, C. D. Matier, A. Bartoszewicz, S. L. Zultanski, J. C. Peters, G. C. Fu, Science 2016, 351, 681–684.
- 24G. C. Fu, ACS Cent. Sci. 2017, 3, 692–700.
- 25Q. Guo, M. Wang, Q. Peng, Y. Huo, Q. Liu, R. Wang, Z. Xu, ACS Catal. 2019, 9, 4470–4476.
- 26F. Wang, D. Wang, X. Wan, L. Wu, P. Chen, G. Liu, J. Am. Chem. Soc. 2016, 138, 15547–15550.
- 27Y. Zhang, Y. Sun, B. Chen, M. Xu, C. Li, D. Zhang, G. Zhang, Org. Lett. 2020, 22, 1490–1494.
- 28Y. Li, K. Zhou, Z. Wen, S. Cao, X. Shen, M. Lei, L. Gong, J. Am. Chem. Soc. 2018, 140, 15850–15858.
- 29B. Han, Y. Li, Y. Yu, L. Gong, Nat. Commun. 2019, 10, 3804.
- 30E. B. McLean, A.-L. Lee, Tetrahedron 2018, 74, 4881–4902.
- 31I. Perepichka, S. Kundu, Z. Hearne, C.-J. Li, Org. Biomol. Chem. 2015, 13, 447–451.
- 32P. Querard, I. Perepichka, E. Zysman-Colman, C.-J. Li, Beilstein J. Org. Chem. 2016, 12, 2636–2643.
- 33F.-D. Lu, D. Liu, L. Zhu, L.-Q. Lu, Q. Yang, Q.-Q. Zhou, Y. Wei, Y. Lan, W.-J. Xiao, J. Am. Chem. Soc. 2019, 141, 6167–6172.
- 34D. Wang, N. Zhu, P. Chen, Z. Lin, G. Liu, J. Am. Chem. Soc. 2017, 139, 15632–15635.
- 35W. Sha, L. Deng, S. Ni, H. Mei, J. Han, Y. Pan, ACS Catal. 2018, 8, 7489–7494.
- 36T. Wang, Y.-N. Wang, R. Wang, B.-C. Zhang, C. Yang, Y.-L. Li, X.-S. Wang, Nat. Commun. 2019, 10, 5373.
- 37J. Chen, P.-Z. Wang, B. Lu, D. Liang, X.-Y. Yu, W.-J. Xiao, J.-R. Chen, Org. Lett. 2019, 21, 9763–9768.
- 38X. Bao, Q. Wang, J. Zhu, Angew. Chem. Int. Ed. 2019, 58, 2139–2143; Angew. Chem. 2019, 131, 2161–2165.
- 39Z. Cheng, P. H. Chen, G. S. Liu, Acta Chim. Sin. (Engl. Ed.) 2019, 77, 856–860.
- 40Y. Li, M. Lei, L. Gong, Nat. Catal. 2019, 2, 1016–1026.
- 41F. Yang, J. Zhao, X. Tang, Y. Wu, Z. Yu, Q. Meng, Adv. Synth. Catal. 2019, 361, 1673–1677.
- 42For selected examples of substrate-controlled photoinduced Ni-catalyzed diastereoselective transformations, please see:
- 42aR. T. Srnith, X. H. Zhang, J. A. Rincon, J. Agejas, C. Mateos, M. Barberis, S. Garcia-Cerrada, O. de Frutos, D. W. C. MacMillan, J. Am. Chem. Soc. 2018, 140, 17433–17438;
- 42bJ. A. Kautzky, T. Wang, R. W. Evans, D. W. C. MacMillan, J. Am. Chem. Soc. 2018, 140, 6522–6526;
- 42cS. O. Badir, A. Dumoulin, J. K. Matsui, G. A. Molander, Angew. Chem. Int. Ed. 2018, 57, 6610–6613; Angew. Chem. 2018, 130, 6720–6723. For selected examples on diastereoselective Cu- or photoredox/Cu-catalyzed transformations, please see:
- 42dA. Hossain, S. Engl, E. Lutsker, O. Reiser, ACS Catal. 2019, 9, 1103–1109;
- 42eT. Rawner, E. Lutsker, C. A. Kaiser, O. Reiser, ACS Catal. 2018, 8, 3950–3956;
- 42fC. Wang, Y. Yu, W.-L. Liu, W.-L. Duan, Org. Lett. 2019, 21, 9147–9152;
- 42gV. O. Smirnov, A. S. Maslov, V. A. Kokorekin, A. A. Korlyukov, A. D. Dilman, Chem. Commun. 2018, 54, 2236–2239;
- 42hX.-W. Gao, Q.-Y. Meng, M. Xiang, B. Chen, K. Feng, C.-H. Tung, L.-Z. Wu, Adv. Synth. Catal. 2013, 355, 2158–2164. For a more general overview of photoinduced Cu-catalyzed transformations, please refer to the review articles cited herein: [22a] and [22e].
- 43S. Zheng, S.-Q. Zhang, B. Saeednia, J. Zhou, J. M. Anna, X. Hong, G. A. Molander, Chem. Sci. 2020, 11, 4131–4137.
- 44G. J. Choi, R. R. Knowles, J. Am. Chem. Soc. 2015, 137, 9226–9229.
- 45A. Dumoulin, J. K. Matsui, A. Gutierrez-Bonet, G. A. Molander, Angew. Chem. Int. Ed. 2018, 57, 6614–6618; Angew. Chem. 2018, 130, 6724–6728.
- 46L. Guo, F. Song, S. Zhu, H. Li, L. Chu, Nat. Commun. 2018, 9, 4543.
- 47C. B. Kelly, N. R. Patel, D. N. Primer, M. Jouffroy, J. C. Tellis, G. A. Molander, Nat. Protoc. 2017, 12, 472–492.
- 48F. Song, F. Wang, L. Guo, X. Feng, Y. Zhang, L. Chu, Angew. Chem. Int. Ed. 2020, 59, 177–181; Angew. Chem. 2020, 132, 183–187.
- 49H. Yue, C. Zhu, R. Kancherla, F. Liu, M. Rueping, Angew. Chem. Int. Ed. 2020, 59, 5738–5746; Angew. Chem. 2020, 132, 5787–5795.
- 50C. Zhu, H. Yue, B. Maity, I. Atodiresei, L. Cavallo, M. Rueping, Nat. Catal. 2019, 2, 678–687.
- 51For selected examples of Cr-catalyzed stereoselective transformations, see:
- 51aJ. L. Schwarz, F. Schäfers, A. Tlahuext-Aca, L. Lückemeier, F. Glorius, J. Am. Chem. Soc. 2018, 140, 12705–12709;
- 51bJ. L. Schwarz, R. Kleinmans, T. O. Paulisch, F. Glorius, J. Am. Chem. Soc. 2020, 142, 2168–2174;
- 51cH. Mitsunuma, S. Tanabe, H. Fuse, K. Ohkubo, M. Kanai, Chem. Sci. 2019, 10, 3459–3465.
Citing Literature
January 25, 2021
Pages 1714-1726