Dealkenylative Alkenylation: Formal σ-Bond Metathesis of Olefins
Dr. Manisha Swain
Department of Chemistry and Biochemistry, University of California—Los Angeles, Los Angeles, CA, 90095-1569 USA
Search for more papers by this authorGusein Sadykhov
Department of Chemistry and Biochemistry, University of California—Los Angeles, Los Angeles, CA, 90095-1569 USA
Search for more papers by this authorRuoxi Wang
Department of Chemistry and Biochemistry, University of California—Los Angeles, Los Angeles, CA, 90095-1569 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Ohyun Kwon
Department of Chemistry and Biochemistry, University of California—Los Angeles, Los Angeles, CA, 90095-1569 USA
Search for more papers by this authorDr. Manisha Swain
Department of Chemistry and Biochemistry, University of California—Los Angeles, Los Angeles, CA, 90095-1569 USA
Search for more papers by this authorGusein Sadykhov
Department of Chemistry and Biochemistry, University of California—Los Angeles, Los Angeles, CA, 90095-1569 USA
Search for more papers by this authorRuoxi Wang
Department of Chemistry and Biochemistry, University of California—Los Angeles, Los Angeles, CA, 90095-1569 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Ohyun Kwon
Department of Chemistry and Biochemistry, University of California—Los Angeles, Los Angeles, CA, 90095-1569 USA
Search for more papers by this authorGraphical Abstract
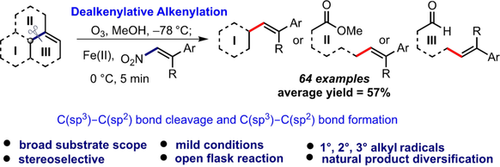
The dealkenylative alkenylation of alkene C(sp3)−C(sp2) bonds has been an unexplored area for C−C bond formation. Reported herein are β-alkylated styrene derivatives synthesized through the reactions of readily accessible feedstock olefins with β-nitrostyrenes by ozone/FeII-mediated radical substitutions. The strategy was applied to the syntheses of the natural product iso-moracin and the drug (E)-metanicotine.
Abstract
The dealkenylative alkenylation of alkene C(sp3)−C(sp2) bonds has been an unexplored area for C−C bond formation. Herein 64 examples of β-alkylated styrene derivatives, synthesized through the reactions of readily accessible feedstock olefins with β-nitrostyrenes by ozone/FeII-mediated radical substitutions, are reported. These reactions proceed with good efficiencies and high stereoselectivities under mild reaction conditions and tolerate an array of functional groups. Also demonstrated is the applicability of the strategy through several synthetic transformations of the products, as well as the syntheses of the natural product iso-moracin and the drug (E)-metanicotine.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202005267-sup-0001-misc_information.pdf11.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Selected reviews:
- 1aS. R. Chemler, D. Trauner, S. J. Danishefsky, Angew. Chem. Int. Ed. 2001, 40, 4544–4568;
10.1002/1521-3773(20011217)40:24<4544::AID-ANIE4544>3.0.CO;2-N CAS PubMed Web of Science® Google ScholarAngew. Chem. 2001, 113, 4676–4701;
- 1bS. E. Denmark, C. R. Butler, Chem. Commun. 2009, 20–33;
- 1cN. A. McGrath, M. Brichacek, J. T. Njardarson, J. Chem. Educ. 2010, 87, 1348–1249;
- 1dC. C. C. Johansson Seechurn, M. O. Kitching, T. J. Colacot, V. Snieck, Angew. Chem. Int. Ed. 2012, 51, 5062–5085; Angew. Chem. 2012, 124, 5150–5174;
- 1eM. B. Smith, J. March, March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th ed., Wiley, Hoboken, 2013;
- 1fR. Álvarez, B. Vaz, H. Gronemeyer, A. R. de Lera, Chem. Rev. 2014, 114, 1–125;
- 1gM. Hassam, A. Taher, G. E. Arnott, I. R. Green, W. A. L. Van Otterlo, Chem. Rev. 2015, 115, 5462–5569.
- 2D. Cristina Silva Costa, Arabian J. Chem. 2020, 13, 799–834.
- 3P. Ertl, T. Schuhmann, J. Nat. Prod. 2019, 82, 1258–1263.
- 4For reviews, see:
- 4aM. Schuster, S. Blechert, Angew. Chem. Int. Ed. Engl. 1997, 36, 2036–2056; Angew. Chem. 1997, 109, 2124–2144;
- 4bO. M. Ogba, N. C. Warner, D. J. O'Leary, R. H. Grubbs, Chem. Soc. Rev. 2018, 47, 4510–4544; For an article describing the synthesis of styrenes, see:
- 4cW. E. Crowe, Z. J. Zhang, J. Am. Chem. Soc. 1993, 115, 10998–10999.
- 5For seminal work, see:
- 5aM. Tsutomu, M. Kunio, O. Atsumu, Bull. Chem. Soc. Jpn. 1971, 44, 581–581;
- 5bR. F. Heck, J. P. Nolley, J. Org. Chem. 1972, 37, 2320–2322;
- 5cR. F. Heck, Acc. Chem. Res. 1979, 12, 146–151; for selected reviews on Heck reaction, see:
- 5dI. P. Beletskaya, A. V. Cheprakov, Chem. Rev. 2000, 100, 3009–3066;
- 5eJ. Ruan, J. Xiao, Acc. Chem. Res. 2011, 44, 614–626;
- 5fJ. Le Bras, J. Muzart, Chem. Rev. 2011, 111, 1170–1214;
- 5gD. Mc Cartney, P. J. Guiry, Chem. Soc. Rev. 2011, 40, 5122–5150.
- 6For reviews, see:
- 6aB. E. Maryanoff, A. B. Reitz, Chem. Rev. 1989, 89, 863–927;
- 6bP. R. Blakemore, J. Chem. Soc. Perkin Trans. 1 2002, 2563–2585;
- 6cP. A. Byrne, D. G. Gilheany, Chem. Soc. Rev. 2013, 42, 6670–6696.
- 7
- 7aR. Shen, T. Chen, Y. Zhao, R. Qiu, Y. Zhou, S. Yin, X. Wang, M. Goto, L.-B. Han, J. Am. Chem. Soc. 2011, 133, 17037–17044;
- 7bS. Fu, N.-Y. Chen, X. Liu, Z. Shao, S.-P. Luo, Q. Liu, J. Am. Chem. Soc. 2016, 138, 8588–8594;
- 7cK. Tokmic, A. R. Fout, J. Am. Chem. Soc. 2016, 138, 13700–13705.
- 8For reviews on Heck-type alkenylations, see:
- 8aS. Tang, K. Liu, C. Liu, A. Lei, Chem. Soc. Rev. 2015, 44, 1070–1082;
- 8bW. Ma, P. Gandeepan, J. Li, L. Ackermann, Org. Chem. Front. 2017, 4, 1435–1467;
- 8cD. Kurandina, P. Chuentragool, V. Gevorgyan, Synthesis 2019, 51, 985–1005.
- 9For reviews on carbonyl-olefination, see:
- 9aL. Ravindar, R. Lekkala, K. P. Rakesh, A. M. Asiri, H. M. Marwani, H.-L. Qin, Org. Chem. Front. 2018, 5, 1381–1391;
- 9bP. S. Riehl, C. S. Schindler, Trends Chem. 2019, 1, 272–273.
- 10Recent reviews on radical chemistry, see:
- 10aA. Studer, D. P. Curran, Angew. Chem. Int. Ed. 2016, 55, 58–102; Angew. Chem. 2016, 128, 58–106;
- 10bH. Yi, G. Zhang, H. Wang, Z. Huang, J. Wang, A. K. Singh, A. Lei, Chem. Rev. 2017, 117, 9016–9085;
- 10cA. Kaga, S. Chiba, ACS Catal. 2017, 7, 4697–4706;
- 10dK. J. Romero, M. S. Galliher, D. A. Pratt, C. R. J. Stephenson, Chem. Soc. Rev. 2018, 47, 7851–7866; For more articles on radicals, see:
- 10eM. Yan, J. C. Lo, J. T. Edwards, P. S. Baran, J. Am. Chem. Soc. 2016, 138, 12692–12714;
- 10fS. Z. Zard, Org. Lett. 2017, 19, 1257–1269.
- 11For review on radical from olefins via HAT, see:
- 11aS. W. M. Crossley, C. Obradors, R. M. Martinez, R. A. Shenvi, Chem. Rev. 2016, 116, 8912–9000; For article, see:
- 11bJ. C. Lo, D. Kim, C.-M. Pan, J. T. Edwards, Y. Yabe, J. Gui, T. Qin, S. Gutiérrez, J. Giacoboni, M. W. Smith, P. L. Holland, P. S. Baran, J. Am. Chem. Soc. 2017, 139, 2484–2503.
- 12
- 12aE. G. E. Hawkins, J. Chem. Soc. 1955, 3463–3467;
- 12bJ. Kumamoto, H. E. De La Mare, F. F. Rust, J. Am. Chem. Soc. 1960, 82, 1935–1939;
- 12cH. E. De La Mare, J. K. Kochi, F. F. Rust, J. Am. Chem. Soc. 1961, 83, 2013;
- 12dS. Murai, N. Sonoda, S. Tsutsumi, Bull. Chem. Soc. Jpn. 1964, 37, 1187–1190;
- 12eS. L. Schreiber, J. Am. Chem. Soc. 1980, 102, 6163–6165.
- 13
- 13aD. Huang, A. W. Schuppe, M. Z. Liang, T. R. Newhouse, Org. Biomol. Chem. 2016, 14, 6197–6200;
- 13bJ. Bao, H. Tian, P. Yang, J. Deng, J. Gui, Eur. J. Org. Chem. 2020, 339–347.
- 14
- 14aA. J. Smaligo, S. Vardhineedi, O. Kwon, ACS Catal. 2018, 8, 5188–5192;
- 14bA. J. Smaligo, M. Swain, J. C. Quintana, M. F. Tan, D. A. Kim, O. Kwon, Science 2019, 364, 681–685;
- 14cA. J. Smaligo, O. Kwon, Org. Lett. 2019, 21, 8592–8597;
- 14dA. J. Smaligo, J. Wu, N. R. Burton, A. S. Hacker, A. C. Shaikh, J. C. Quintana, R. Wang, C. Xie, O. Kwon, Angew. Chem. Int. Ed. 2020, 59, 1211–1215; Angew. Chem. 2020, 132, 1227–1231.
- 15R. Criegee, G. Wenner, Justus Liebigs Ann. Chem. 1949, 564, 9–15.
- 16
- 16aB. M. Fox, X. S. Xiao, S. Antony, G. Kohlhagen, Y. Pommier, B. L. Staker, L. Stewart, M. Cushman, J. Med. Chem. 2003, 46, 3275–3282;
- 16bJ. Cheel, C. Theoduloz, J. Rodríguez, G. Saud, P. D. S. Caligari, G. Schmeda-Hirschmann, J. Agric. Food Chem. 2005, 53, 8512–8518;
- 16cB. S. Siddiqui, H. Aslam, S. Begum, S. T. Ali, Nat. Prod. Res. 2007, 21, 736–741;
- 16dY. Lee, B. Park, W. Lyoo, Synthesis 2009, 2146–2154;
- 16eP.-H. Nguyen, J.-L. Yang, M. N. Uddin, S.-L. Park, S.-I. Lim, D.-W. Jung, D. R. Williams, W.-K. Oh, J. Nat. Prod. 2013, 76, 2080–2087.
- 17
- 17aK. Patel, C. Karthikeyan, N. S. H. N. Moorthy, G. S. Deora, V. R. Solomon, H. Lee, P. Trivedi, Med. Chem. Res. 2012, 21, 1780–1784;
- 17bN. Sharma, D. Mohanakrishnan, A. Shard, A. Sharma, A. K. Sinha, D. Sahal, J. Med. Chem. 2012, 55, 297–311;
- 17cR. S. P. Singh, D. Michel, U. Das, J. R. Dimmock, J. Alcorn, Bioorg. Med. Chem. Lett. 2014, 24, 5199–5202.
- 18A. Noble, S. J. McCarver, D. W. C. MacMillan, J. Am. Chem. Soc. 2015, 137, 624–627.
- 19
- 19aZ. Cui, X. Shang, X.-F. Shao, Z.-Q. Liu, Chem. Sci. 2012, 3, 2853–2858;
- 19bJ. Zhao, H. Fang, J. Han, Y. Pan, Beilstein J. Org. Chem. 2013, 9, 1718–1723;
- 19cW.-P. Mai, G. Song, G.-C. Sun, L.-R. Yang, J.-W. Yuan, Y.-M. Xiao, P. Mao, L.-B. Qu, RSC Adv. 2013, 3, 19264–19267;
- 19dJ. Ji, P. Liu, P. Sun, Chem. Commun. 2015, 51, 7546–7549;
- 19eZ. Fang, C. Wei, J. Lin, Z. Liu, W. Wang, C. Xu, X. Wang, Y. Wang, Org. Biomol. Chem. 2017, 15, 9974–9977;
- 19fC. Wang, Y. Lei, M. Guo, Q. Shang, H. Liu, Z. Xu, R. Wang, Org. Lett. 2017, 19, 6412–6415;
- 19gR. Kancherla, K. Muralirajan, B. Maity, C. Zhu, P. E. Krach, L. Cavallo, M. Rueping, Angew. Chem. Int. Ed. 2019, 58, 3412–3416; Angew. Chem. 2019, 131, 3450–3454.
- 20
- 20aJ.-Y. Liu, J.-T. Liu, C.-F. Yao, Tetrahedron Lett. 2001, 42, 3613–3615;
- 20bJ.-T. Liu, Y.-J. Jang, Y.-K. Shih, S.-R. Hu, C.-M. Chu, C.-F. Yao, J. Org. Chem. 2001, 66, 6021–6028;
- 20cY.-J. Jang, Y.-K. Shih, J.-Y. Liu, W.-Y. Kuo, C.-F. Yao, Chem. Eur. J. 2003, 9, 2123–2128;
- 20dY.-J. Jang, M.-C. Yan, Y.-F. Lin, C.-F. Yao, J. Org. Chem. 2004, 69, 3961–3963;
- 20eS.-r. Guo, Y.-q. Yuan, Synlett 2015, 26, 1961–1968;
- 20fJ. Zheng, D. Wang, S. Cui, Org. Lett. 2015, 17, 4572–4575;
- 20gS. Zhang, Y. Li, J. Wang, X. Hao, K. Jin, R. Zhang, C. Duan, Tetrahedron Lett. 2020, 61, 151721.
- 21
- 21aA. Noble, D. W. C. MacMillan, J. Am. Chem. Soc. 2014, 136, 11602–11605;
- 21bS. Sumino, M. Uno, H.-J. Huang, Y.-K. Wu, I. Ryu, Org. Lett. 2018, 20, 1078–1081;
- 21cQ.-Q. Zhou, S. J. S. Düsel, L.-Q. Lu, B. König, W.-J. Xiao, Chem. Commun. 2019, 55, 107–110.
- 22A. Boelke, L. D. Caspers, B. J. Nachtsheim, Org. Lett. 2017, 19, 5344–5347.
- 23
- 23aJ. K. Kochi, J. Am. Chem. Soc. 1962, 84, 1193–1197;
- 23bW. H. Richardson, J. Org. Chem. 1965, 30, 2804–2808;
- 23cR. R. Hiatt, K. C. Irwin, C. W. Gould, J. Org. Chem. 1968, 33, 1430–1435.
- 24
- 24aS. Alakurtti, T. Mäkelä, S. Koskimies, J. Yli-Kauhaluoma, Eur. J. Pharm. Sci. 2006, 29, 1–13;
- 24bM. Grymel, M. Zayozak, J. Adamek, J. Nat. Prod. 2019, 82, 1719–1730.
- 25
- 25aB. Giese, Angew. Chem. Int. Ed. Engl. 1989, 28, 969–980; Angew. Chem. 1989, 101, 993–1004;
- 25bG. Bar, A. F. Parsons, Chem. Soc. Rev. 2003, 32, 251–263.
- 26
- 26aK. L. Jensen, G. Dickmeiss, B. S. Donslund, P. H. Poulsen, K. A. Jørgensen, Org. Lett. 2011, 13, 3678–3681;
- 26bJ. Petrignet, A. Boudhar, G. Blond, J. Suffer, Angew. Chem. Int. Ed. 2011, 50, 3285–3289; Angew. Chem. 2011, 123, 3343–3347.
- 27For reviews on the halogenation of olefins, see:
- 27aG. Chen, S. Ma, Angew. Chem. Int. Ed. 2010, 49, 8306–8308; Angew. Chem. 2010, 122, 8484–8486;
- 27bA. Castellanos, S. P. Fletcher, Chem. Eur. J. 2011, 17, 5766–5776;
- 27cS. C. Snyder, D. S. Treitler, A. P. Brucks, Aldrichimica Acta 2011, 44, 27–40;
- 27dU. Hennecke, Chem. Asian J. 2012, 7, 456–465;
- 27eS. E. Denmark, W. E. Kuester, M. T. Burk, Angew. Chem. Int. Ed. 2012, 51, 10938–10953; Angew. Chem. 2012, 124, 11098–11113.
- 28X. Zeng, C. Miao, S. Wang, C. Xia, W. Sun, Synthesis 2013, 45, 2391–2396.
- 29Y. Sakata, E. Yasui, K. Takatori, Y. Suzuki, M. Mizukami, S. Nagumo, J. Org. Chem. 2018, 83, 9103–9118.
- 30
- 30aY. M. Syah, E. H. Hakim, L. Makmur, V. A. Kurdi, E. L. Ghisalberti, N. Aimi, S. A. Achmad, Nat. Prod. Commun. 2006, 1, 549–552;
- 30bX. Li, H. Xie, R. Zhan, D. Chen, Molecules 2018, 23, 754–764.
- 31D. Wu, H. Mei, P. Tan, W. Lu, J. Zhu, W. Wang, J. Huan, J. Li, Tetrahedron Lett. 2015, 56, 4383–4387.
- 32J. Jang, K. S. Sin, H. Park, Arch. Pharmacal Res. 2001, 24, 503–507.
- 33
- 33aM. Bencherif, M. E. Lovette, K. W. Fowler, S. Arrington, L. Reeves, W. S. Caldwell, P. M. Lippiello, J. Pharmacol. Exp. Ther. 1996, 279, 1413–1421;
- 33bN. S. Sapronov, Y. O. Fedotova, N. N. Kuznetsova, Bull. Exp. Biol. Med. 2006, 142, 700–702.
- 34CCDC 1986086 (7 ab) and 1986087 (16) contain the supplementary crystallographic data for this paper. These data are provided free of charge by The Cambridge Crystallographic Data Centre.