Single-Atom Iron Catalysts on Overhang-Eave Carbon Cages for High-Performance Oxygen Reduction Reaction
Dr. Chun-Chao Hou
AIST-Kyoto University Chemical Energy Materials Open Innovation Laboratory (ChEM-OIL), National Institute of Advanced Industrial Science and Technology (AIST), Sakyo-ku, Kyoto, 606-8501 Japan
Search for more papers by this authorDr. Lianli Zou
AIST-Kyoto University Chemical Energy Materials Open Innovation Laboratory (ChEM-OIL), National Institute of Advanced Industrial Science and Technology (AIST), Sakyo-ku, Kyoto, 606-8501 Japan
Graduate School of Engineering, Kobe University, Nada Ku, Kobe, Hyogo, 657-8501 Japan
Search for more papers by this authorDr. Liming Sun
AIST-Kyoto University Chemical Energy Materials Open Innovation Laboratory (ChEM-OIL), National Institute of Advanced Industrial Science and Technology (AIST), Sakyo-ku, Kyoto, 606-8501 Japan
Laboratory of Quantum Materials Design and Application, School of Physics and Electronic Engineering, Jiangsu Normal University, Xuzhou, 221116 P. R. China
Search for more papers by this authorDr. Kexin Zhang
Beijing Key laboratory for Theory and Technology of Advanced Battery Materials, Department of Materials Science and Engineering, College of Engineering, Peking University, Beijing, 100871 P. R. China
Search for more papers by this authorDr. Zheng Liu
Inorganic Functional Materials Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), 2266-98 Anagahora, Shimoshidami, Moriyamaku, Nagoya, Aichi, 463-8560 Japan
Search for more papers by this authorProf. Yinwei Li
Laboratory of Quantum Materials Design and Application, School of Physics and Electronic Engineering, Jiangsu Normal University, Xuzhou, 221116 P. R. China
Search for more papers by this authorDr. Caixia Li
AIST-Kyoto University Chemical Energy Materials Open Innovation Laboratory (ChEM-OIL), National Institute of Advanced Industrial Science and Technology (AIST), Sakyo-ku, Kyoto, 606-8501 Japan
Search for more papers by this authorProf. Ruqiang Zou
Beijing Key laboratory for Theory and Technology of Advanced Battery Materials, Department of Materials Science and Engineering, College of Engineering, Peking University, Beijing, 100871 P. R. China
Search for more papers by this authorProf. Jihong Yu
State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry; International Center of Future Science, Jilin University, Changchun, 130012 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Qiang Xu
AIST-Kyoto University Chemical Energy Materials Open Innovation Laboratory (ChEM-OIL), National Institute of Advanced Industrial Science and Technology (AIST), Sakyo-ku, Kyoto, 606-8501 Japan
Graduate School of Engineering, Kobe University, Nada Ku, Kobe, Hyogo, 657-8501 Japan
Search for more papers by this authorDr. Chun-Chao Hou
AIST-Kyoto University Chemical Energy Materials Open Innovation Laboratory (ChEM-OIL), National Institute of Advanced Industrial Science and Technology (AIST), Sakyo-ku, Kyoto, 606-8501 Japan
Search for more papers by this authorDr. Lianli Zou
AIST-Kyoto University Chemical Energy Materials Open Innovation Laboratory (ChEM-OIL), National Institute of Advanced Industrial Science and Technology (AIST), Sakyo-ku, Kyoto, 606-8501 Japan
Graduate School of Engineering, Kobe University, Nada Ku, Kobe, Hyogo, 657-8501 Japan
Search for more papers by this authorDr. Liming Sun
AIST-Kyoto University Chemical Energy Materials Open Innovation Laboratory (ChEM-OIL), National Institute of Advanced Industrial Science and Technology (AIST), Sakyo-ku, Kyoto, 606-8501 Japan
Laboratory of Quantum Materials Design and Application, School of Physics and Electronic Engineering, Jiangsu Normal University, Xuzhou, 221116 P. R. China
Search for more papers by this authorDr. Kexin Zhang
Beijing Key laboratory for Theory and Technology of Advanced Battery Materials, Department of Materials Science and Engineering, College of Engineering, Peking University, Beijing, 100871 P. R. China
Search for more papers by this authorDr. Zheng Liu
Inorganic Functional Materials Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), 2266-98 Anagahora, Shimoshidami, Moriyamaku, Nagoya, Aichi, 463-8560 Japan
Search for more papers by this authorProf. Yinwei Li
Laboratory of Quantum Materials Design and Application, School of Physics and Electronic Engineering, Jiangsu Normal University, Xuzhou, 221116 P. R. China
Search for more papers by this authorDr. Caixia Li
AIST-Kyoto University Chemical Energy Materials Open Innovation Laboratory (ChEM-OIL), National Institute of Advanced Industrial Science and Technology (AIST), Sakyo-ku, Kyoto, 606-8501 Japan
Search for more papers by this authorProf. Ruqiang Zou
Beijing Key laboratory for Theory and Technology of Advanced Battery Materials, Department of Materials Science and Engineering, College of Engineering, Peking University, Beijing, 100871 P. R. China
Search for more papers by this authorProf. Jihong Yu
State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry; International Center of Future Science, Jilin University, Changchun, 130012 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Qiang Xu
AIST-Kyoto University Chemical Energy Materials Open Innovation Laboratory (ChEM-OIL), National Institute of Advanced Industrial Science and Technology (AIST), Sakyo-ku, Kyoto, 606-8501 Japan
Graduate School of Engineering, Kobe University, Nada Ku, Kobe, Hyogo, 657-8501 Japan
Search for more papers by this authorGraphical Abstract
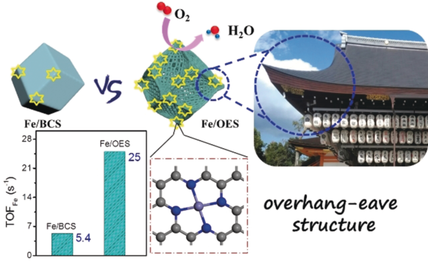
An overhang-eave carbon cage decorated with single-atom iron catalysts for oxygen reduction (ORR) is prepared. This structure could furnish more stretched edges as three-phase boundaries to support the mass transport of ORR-relevant species and to expose the atomically dispersed/catalytically active iron sites to the utmost, thus increasing the utilization of each active site.
Abstract
Single-atom catalysts have drawn great attention, especially in electrocatalysis. However, most of previous works focus on the enhanced catalytic properties via improving metal loading. Engineering morphologies of catalysts to facilitate mass transport through catalyst layers, thus increasing the utilization of each active site, is regarded as an appealing way for enhanced performance. Herein, we design an overhang-eave structure decorated with isolated single-atom iron sites via a silica-mediated MOF-templated approach for oxygen reduction reaction (ORR) catalysis. This catalyst demonstrates superior ORR performance in both alkaline and acidic electrolytes, comparable to the state-of-the-art Pt/C catalyst and superior to most precious-metal-free catalysts reported to date. This activity originates from its edge-rich structure, having more three-phase boundaries with enhanced mass transport of reactants to accessible single-atom iron sites (increasing the utilization of active sites), which verifies the practicability of such a synthetic approach.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202002665-sup-0001-misc_information.pdf4.8 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Y. Chen, S. Ji, C. Chen, Q. Peng, D. Wang, Y. Li, Joule 2018, 2, 1242–1264.
- 2B.-H. Lee, S. Park, M. Kim, A. K. Sinha, S. C. Lee, E. Jung, W. J. Chang, K.-S. Lee, J. H. Kim, S.-P. Cho, H. Kim, K. T. Nam, T. Hyeon, Nat. Mater. 2019, 18, 620–626.
- 3C.-C. Hou, L. Zou, Q. Xu, Adv. Mater. 2019, 31, 1904689.
- 4
- 4aY. Zhang, J. Sokolowski, X. Song, Y. He, Y. Mei, G. Wu, Adv. Energy Mater. 2020, https://doi.org/10.1002/aenm.201902844;
- 4bA. Wang, J. Li, T. Zhang, Nat. Rev. Chem. 2018, 2, 65–81.
- 5L. Jiao, H.-L. Jiang, Chem 2019, 5, 786–804.
- 6D.-S. Bin, Z.-X. Chi, Y. Li, K. Zhang, X. Yang, Y.-G. Sun, J.-Y. Piao, A.-M. Cao, L.-J. Wan, J. Am. Chem. Soc. 2017, 139, 13492–13498.
- 7Y.-G. Sun, J.-Y. Piao, L.-L. Hu, D.-S. Bin, X.-J. Lin, S.-Y. Duan, A.-M. Cao, L.-J. Wan, J. Am. Chem. Soc. 2018, 140, 9070–9073.
- 8Y. Jiao, Y. Zheng, K. Davey, S.-Z. Qiao, Nat. Energy 2016, 1, 16130.
- 9H. Li, M. Eddaoudi, M. O'Keeffe, O. M. Yaghi, Nature 1999, 402, 276–279.
- 10
- 10aC.-C. Hou, Q. Xu, Adv. Energy Mater. 2019, 9, 1801307;
- 10bB. Liu, H. Shioyama, T. Akita, Q. Xu, J. Am. Chem. Soc. 2008, 130, 5390–5391;
- 10cZ. Liang, R. Zhao, T. Qiu, R. Zou, Q. Xu, EnergyChem 2019, 1, 100001.
10.1016/j.enchem.2019.100001 Google Scholar
- 11R. Zhao, Z. Liang, S. Cao, C. Yang, B. Zhu, J. Zhao, C. Qu, R. Zou, Q. Xu, Angew. Chem. Int. Ed. 2019, 58, 1975–1979; Angew. Chem. 2019, 131, 1997–2001.
- 12Y. He, S. Hwang, D. A. Cullen, M. A. Uddin, L. Langhorst, B. Li, S. Karakalos, A. J. Kropf, E. C. Wegener, J. Sokolowski, M. Chen, D. Myers, D. Su, K. L. More, G. Wang, S. Litster, G. Wu, Energy Environ. Sci. 2019, 12, 250–260.
- 13X. X. Wang, M. T. Swihart, G. Wu, Nat. Catal. 2019, 2, 578–589.
- 14
- 14aH.-F. Wang, L. Chen, H. Pang, S. Kaskel, Q. Xu, Chem. Soc. Rev. 2020, 49, 1414–1448;
- 14bC. Wang, J. Kim, J. Tang, M. Kim, H. Lim, V. Malgras, J. You, Q. Xu, J. Li, Y. Yamauchi, Chem 2020, 6, 19–40;
- 14cS. Gadipelli, T. Zhao, S. A. Shevlin, Z. Guo, Energy Environ. Sci. 2016, 9, 1661–1667.
- 15
- 15aC. Hu, L. Dai, Angew. Chem. Int. Ed. 2016, 55, 11736–11758; Angew. Chem. 2016, 128, 11910–11933;
- 15bL. Zou, C.-C. Hou, Z. Liu, H. Pang, Q. Xu, J. Am. Chem. Soc. 2018, 140, 15393–15401;
- 15cA. A. Gewirth, J. A. Varnell, A. M. DiAscro, Chem. Rev. 2018, 118, 2313–2339.
- 16
- 16aJ. Wang, Z. Huang, W. Liu, C. Chang, H. Tang, Z. Li, W. Chen, C. Jia, T. Yao, S. Wei, Y. Wu, Y. Li, J. Am. Chem. Soc. 2017, 139, 17281–17284;
- 16bY. Chen, S. Ji, S. Zhao, W. Chen, J. Dong, W.-C. Cheong, R. Shen, X. Wen, L. Zheng, A. I. Rykov, S. Cai, H. Tang, Z. Zhuang, C. Chen, Q. Peng, D. Wang, Y. Li, Nat. Commun. 2018, 9, 5422.
- 17K. S. Park, Z. Ni, A. P. Côté, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe, O. M. Yaghi, Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191.
- 18L. Shang, H. Yu, X. Huang, T. Bian, R. Shi, Y. Zhao, G. I. N. Water-house, L. Z. Wu, C. H. Tung, T. R. Zhang, Adv. Mater. 2016, 28, 1668–1674.
- 19
- 19aJ. Han, X. Meng, L. Lu, J. Bian, Z. Li, C. Sun, Adv. Funct. Mater. 2019, 29, 1808872;
- 19bL. Jiao, G. Wan, R. Zhang, H. Zhou, S.-H. Yu, H.-L. Jiang, Angew. Chem. Int. Ed. 2018, 57, 8525–8529; Angew. Chem. 2018, 130, 8661–8665;
- 19cW. Liu, L. Zhang, X. Liu, X. Liu, X. Yang, S. Miao, W. Wang, A. Wang, T. Zhang, J. Am. Chem. Soc. 2017, 139, 10790–10798.
- 20H. Zhang, S. Hwang, M. Wang, Z. Feng, S. Karakalos, L. Luo, Z. Qiao, X. Xie, C. Wang, D. Su, Y. Shao, G. Wu, J. Am. Chem. Soc. 2017, 139, 14143–14149.
- 21
- 21aC. Zhu, Q. Shi, B. Z. Xu, S. Fu, G. Wan, C. Yang, S. Yao, J. Song, H. Zhou, D. Du, S. P. Beckman, D. Su, Y. Lin, Adv. Energy Mater. 2018, 8, 1801956;
- 21bY. Chen, S. Ji, Y. Wang, J. Dong, W. Chen, Z. Li, R. Shen, L. Zheng, Z. Zhuang, D. Wang, Y. Li, Angew. Chem. Int. Ed. 2017, 56, 6937–6941; Angew. Chem. 2017, 129, 7041–7045.
- 22Q. Zhu, W. Xia, T. Akita, R. Zou, Q. Xu, Adv. Mater. 2016, 28, 6391–6398.
- 23M. Xiao, J. Zhu, L. Ma, Z. Jin, J. Ge, X. Deng, Y. Hou, Q. He, J. Li, Q. Jia, S. Mukerjee, R. Yang, Z. Jiang, D. Su, C. Liu, W. Xing, ACS Catal. 2018, 8, 2824–2832.
- 24
- 24aS. Liu, Z. Wang, S. Zhou, F. Yu, M. Yu, C.-Y. Chiang, W. Zhou, J. Zhao, J. Qiu, Adv. Mater. 2017, 29, 1700874;
- 24bC.-C. Hou, S. Cao, W.-F. Fu, Y. Chen, ACS Appl. Mater. Interfaces 2015, 7, 28412–28419.
- 25Y. V. Kaneti, J. Tang, R. R. Salunkhe, X. Jiang, A. Yu, K. C.-W. Wu, Y. Yamauchi, Adv. Mater. 2017, 29, 1604898.
- 26
- 26aL. Tong, Y.-C. Wang, M.-X. Chen, Z.-Q. Chen, Q.-Q. Yan, C.-L. Yang, Z.-Y. Zhou, S.-Q. Chu, X. Feng, H.-W. Liang, Chem. Sci. 2019, 10, 8236–8240;
- 26bL. Zou, M. Kitta, J. Hong, K. Suenaga, N. Tsumori, Z. Liu, Q. Xu, Adv. Mater. 2019, 31, 1900440.
- 27
- 27aS. J. Yang, T. Kim, J. H. Im, Y. S. Kim, K. Lee, H. Jung, C. R. Park, Chem. Mater. 2012, 24, 464–470;
- 27bH. Huang, M. Yan, C. Yang, H. He, Q. Jiang, L. Yang, Z. Lu, Z. Sun, X. Xu, Y. Bando, Y. Yamauchi, Adv. Mater. 2019, 31, 1903415.
- 28Y. Pan, S. Liu, K. Sun, X. Chen, B. Wang, K. Wu, X. Cao, W. Cheong, R. Shen, A. Han, Z. Chen, L. Zheng, J. Luo, Y. Lin, Y. Liu, D. Wang, Q. Peng, Q. Zhang, C. Chen, Y. Li, Angew. Chem. Int. Ed. 2018, 57, 8614–8618; Angew. Chem. 2018, 130, 8750–8754.
- 29Y. Hou, M. Qiu, T. Zhang, J. Ma, S. Liu, X. Zhuang, C. Yuan, X. Feng, Adv. Mater. 2017, 29, 1604480.
- 30W. Zhang, Z.-Y. Wu, H.-L. Jiang, S.-H. Yu, J. Am. Chem. Soc. 2014, 136, 14385–14388.
- 31S. H. Lee, J. Kim, D. Y. Chuang, J. M. Yoo, H. S. Lee, M. J. Kim, B. S. Mun, S. G. Kwon, Y.-E. Sung, T. Hyeon, J. Am. Chem. Soc. 2019, 141, 2035–2045.
- 32A. J. Bard, L. R. Faulkner, Electrochemical Methods: Fundamentals and Applications, Wiley, New York, 2001.
- 33R. Zhou, Y. Zheng, M. Jaroniec, S.-Z. Qiao, ACS Catal. 2016, 6, 4720–4728.
- 34
- 34aJ. Pan, Y. Y. Xu, H. Yang, Z. Dong, H. Liu, B. Y. Xia, Adv. Sci. 2018, 5, 1700691;
- 34bC. Tang, B. Wang, H. F. Wang, Q. Zhang, Adv. Mater. 2017, 29, 1703185.
- 35J. Zhang, Q. Zhou, Y. Tang, L. Zhang, Y. Li, Chem. Sci. 2019, 10, 8924–8929.
- 36S. Zhu, J. Ge, C. Liu, W. Xing, EnergyChem 2019, 1, 100018.
10.1016/j.enchem.2019.100018 Google Scholar
- 37U. I. Kramm, J. Herranz, N. Larouche, T. M. Arruda, M. Lefèvre, F. Jaouen, P. Bogdanoff, S. Fiechter, I. Abs-Wurmbach, S. Mukerjee, J.-P. Dodelet, Phys. Chem. Chem. Phys. 2012, 14, 11673–11688.
- 38H. Zhang, H. T. Chung, D. A. Cullen, S. Wagner, U. I. Kramm, K. L. More, P. Zelenay, G. Wu, Energy Environ. Sci. 2019, 12, 2548–2558.
- 39A. Zitolo, V. Goellner, V. Armel, M.-T. Sougrati, T. Mineva, L. Stievano, E. Fonda, F. Jaouen, Nat. Mater. 2015, 14, 937–942.
- 40M. Xiao, J. Zhu, G. Li, N. Li, S. Li, Z. P. Cano, L. Ma, P. Cui, P. Xu, G. Jiang, H. Jin, S. Wang, T. Wu, J. Lu, A. Yu, D. Su, Z. Chen, Angew. Chem. Int. Ed. 2019, 58, 9640–9645; Angew. Chem. 2019, 131, 9742–9747.