Endogenous Nanoparticles Strain Perovskite Host Lattice Providing Oxygen Capacity and Driving Oxygen Exchange and CH4 Conversion to Syngas
Graphical Abstract
Abstract
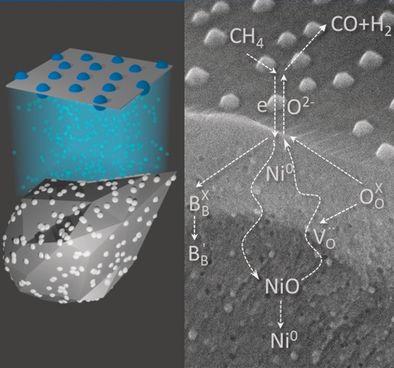
Particles dispersed on the surface of oxide supports have enabled a wealth of applications in electrocatalysis, photocatalysis, and heterogeneous catalysis. Dispersing nanoparticles within the bulk of oxides is, however, synthetically much more challenging and therefore less explored, but could open new dimensions to control material properties analogous to substitutional doping of ions in crystal lattices. Here we demonstrate such a concept allowing extensive, controlled growth of metallic nanoparticles, at nanoscale proximity, within a perovskite oxide lattice as well as on its surface. By employing operando techniques, we show that in the emergent nanostructure, the endogenous nanoparticles and the perovskite lattice become reciprocally strained and seamlessly connected, enabling enhanced oxygen exchange. Additionally, even deeply embedded nanoparticles can reversibly exchange oxygen with a methane stream, driving its redox conversion to syngas with remarkable selectivity and long term cyclability while surface particles are present. These results not only exemplify the means to create extensive, self-strained nanoarchitectures with enhanced oxygen transport and storage capabilities, but also demonstrate that deeply submerged, redox-active nanoparticles could be entirely accessible to reaction environments, driving redox transformations and thus offering intriguing new alternatives to design materials underpinning several energy conversion technologies.
Conflict of interest
The authors declare no conflict of interest.