1,1-Diphenylvinylsulfide as a Functional AIEgen Derived from the Aggregation-Caused-Quenching Molecule 1,1-Diphenylethene through Simple Thioetherification
Bo-Wen Wang
School of Chemistry, South China Normal University, Key Laboratory of Theoretical Chemistry of Environment, Ministry of Education, Guangzhou, 510006 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorKai Jiang
School of Chemistry, South China Normal University, Key Laboratory of Theoretical Chemistry of Environment, Ministry of Education, Guangzhou, 510006 P. R. China
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, 510640 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorDr. Jian-Xiao Li
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, 510640 P. R. China
Search for more papers by this authorDr. Shi-He Luo
School of Chemistry, South China Normal University, Key Laboratory of Theoretical Chemistry of Environment, Ministry of Education, Guangzhou, 510006 P. R. China
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, 510640 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhao-Yang Wang
School of Chemistry, South China Normal University, Key Laboratory of Theoretical Chemistry of Environment, Ministry of Education, Guangzhou, 510006 P. R. China
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, 510640 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Huan-Feng Jiang
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, 510640 P. R. China
Search for more papers by this authorBo-Wen Wang
School of Chemistry, South China Normal University, Key Laboratory of Theoretical Chemistry of Environment, Ministry of Education, Guangzhou, 510006 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorKai Jiang
School of Chemistry, South China Normal University, Key Laboratory of Theoretical Chemistry of Environment, Ministry of Education, Guangzhou, 510006 P. R. China
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, 510640 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorDr. Jian-Xiao Li
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, 510640 P. R. China
Search for more papers by this authorDr. Shi-He Luo
School of Chemistry, South China Normal University, Key Laboratory of Theoretical Chemistry of Environment, Ministry of Education, Guangzhou, 510006 P. R. China
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, 510640 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhao-Yang Wang
School of Chemistry, South China Normal University, Key Laboratory of Theoretical Chemistry of Environment, Ministry of Education, Guangzhou, 510006 P. R. China
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, 510640 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Huan-Feng Jiang
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, 510640 P. R. China
Search for more papers by this authorGraphical Abstract
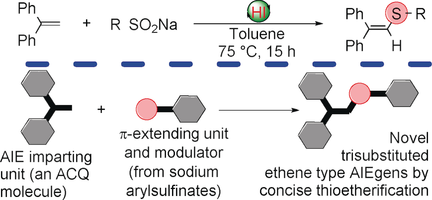
A simple thioetherification between 1,1-diphenylethene as an aggregation-induced emission (AIE)-imparting unit and sodium sulfinates provides a new route for the conversion of compounds with aggregation-caused quenching (ACQ) properties into AIE luminogens. This reaction delivers up to 99 % yield under mild conditions without additional oxidant, and gives access to novel trisubstituted-ethene-type AIEgens with potential for use in sensing applications.
Abstract
An efficient and readily scalable thioetherification between 1,1-diphenylethene (DPE) and sodium arylsulfinate was developed for the synthesis of 1,1-diphenylvinylsulfide (DPVS) with the yield up to 99 %. The photophysical properties of DPVS show that the introduction of arylsulfenyl groups onto the parent molecule DPE makes DPVS a novel type of aggregation-induced emission (AIE) luminogen (AIEgen) with large Stoke's shift (up to 188 nm). These DPVS possess AIE properties due to restriction of intramolecular motions (RIM), as demonstrated by crystal structure analysis. Importantly, the AIE performance of DPVS can be applied to sense the nitroaromatic explosive picric acid in aqueous systems through a “turn-off” response.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201914333-sup-0001-misc_information.pdf10.6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. D. Luo, Z. L. Xie, J. W. Y. Lam, L. Cheng, H. Y. Chen, C. F. Qiu, H. S. Kwok, X. W. Zhan, Y. Q. Liu, D. B. Zhu, B. Z. Tang, Chem. Commun. 2001, 1740–1741;
- 1bY. N. Hong, J. W. Y. Lam, B. Z. Tang, Chem. Commun. 2009, 4332–4353;
- 1cR. R. Hu, N. L. C. Leung, B. Z. Tang, Chem. Soc. Rev. 2014, 43, 4494–4562;
- 1dP. C. Shen, Z. Y. Zhuang, Z. J. Zhao, B. Z. Tang, J. Mater. Chem. C 2018, 6, 11835–11852;
- 1eD. Wang, M. M. S. Lee, W. H. Xu, G. G. Shan, X. Y. Zheng, R. T. K. Kwok, J. W. Y. Lam, X. L. Hu, B. Z. Tang, Angew. Chem. Int. Ed. 2019, 58, 5628–5632; Angew. Chem. 2019, 131, 5684–5688;
- 1fY. J. Tu, J. K. Liu, H. K. Zhang, Q. Peng, J. W. Y. Lam, B. Z. Tang, Angew. Chem. Int. Ed. 2019, 58, 14911–14914; Angew. Chem. 2019, 131, 15053–15056;
- 1gG. X. Huang, Q. Xia, W. B. Huang, J. W. Tian, Z. K. He, B. S. Li, B. Z. Tang, Angew. Chem. Int. Ed. 2019, 58, 17814–17819; Angew. Chem. 2019, 131, 17978–17983.
- 2
- 2aB.-K. An, S.-K. Kwon, S.-D. Jung, S. Y. Park, J. Am. Chem. Soc. 2002, 124, 14410–14415;
- 2bS.-J. Yoon, J. W. Chung, J. Gierschner, K. S. Kim, M.-G. Choi, D. Kim, S. Y. Park, J. Am. Chem. Soc. 2010, 132, 13675–13683;
- 2cB.-K. An, J. Gierschner, S. Y. Park, Acc. Chem. Res. 2012, 45, 544–554.
- 3
- 3aZ. G. Chi, X. Q. Zhang, B. J. Xu, X. Zhou, C. P. Ma, Y. Zhang, S. W. Liu, J. R. Xu, Chem. Soc. Rev. 2012, 41, 3878–3896;
- 3bY.-L. Ying, Y.-J. Li, J. Mei, R. Gao, Y.-X. Hu, Y.-T. Long, H. Tian, Nat. Commun. 2018, 9, 3657;
- 3cY. S. Ren, S. Xie, E. S. Grape, A. K. Inge, O. Ramström, J. Am. Chem. Soc. 2018, 140, 13640–13643;
- 3dC. F. Wang, X. Wang, P. P. Gai, H. Y. Li, F. Li, Sens. Actuators B 2019, 284, 118–124;
- 3eN. Zhao, P. F. Li, J. B. Zhuang, Y. Y. Liu, Y. X. Xiao, R. L. Qin, N. Li, ACS Appl. Mater. Interfaces 2019, 11, 11227–11237;
- 3fG. J. Tian, D. X. Sun, Y. G. Zhang, X. Yu, Angew. Chem. Int. Ed. 2019, 58, 5951–5955; Angew. Chem. 2019, 131, 6012–6016;
- 3gK. Kokado, K. Sada, Angew. Chem. Int. Ed. 2019, 58, 8632–8639; Angew. Chem. 2019, 131, 8724–8731.
- 4J. Mei, Y. N. Hong, J. W. Y. Lam, A. J. Qin, Y. H. Tang, B. Z. Tang, Adv. Mater. 2014, 26, 5429–5479.
- 5C.-L. Chiang, S.-M. Tseng, C.-T. Chen, C.-P. Hsu, C.-F. Shu, Adv. Funct. Mater. 2008, 18, 248–257.
- 6
- 6aS. J. Liu, Y. H. Cheng, H. K. Zhang, Z. J. Qiu, R. T. K. Kwok, J. W. Y. Lam, B. Z. Tang, Angew. Chem. Int. Ed. 2018, 57, 6274–6278; Angew. Chem. 2018, 130, 6382–6386;
- 6bH.-T. Feng, Y.-X. Yuan, J.-B. Xiong, Y.-S. Zheng, B. Z. Tang, Chem. Soc. Rev. 2018, 47, 7452–7476.
- 7Z. J. Zhao, B. R. He, B. Z. Tang, Chem. Sci. 2015, 6, 5347–5365.
- 8J. Huang, N. Sun, Y. Q. Dong, R. L. Tang, P. Lu, P. Cai, Q. Q. Li, D. G. Ma, J. G. Qin, Z. Li, Adv. Funct. Mater. 2013, 23, 2329–2337.
- 9
- 9aD. Ding, K. Li, B. Liu, B. Z. Tang, Acc. Chem. Res. 2013, 46, 2441–2453;
- 9bM. J. Jiang, X. G. Gu, R. T. K. Kwok, Y. Li, H. H. Y. Sung, X. Zheng, Y. L. Zhang, J. W. Y. Lam, I. D. Williams, X. H. Huang, K. S. Wong, B. Z. Tang, Adv. Funct. Mater. 2018, 28, 1704589;
- 9cD. D. He, Z. Y. Zhuang, X. Wang, J. W. Li, J. X. Li, W. Q. Wu, Z. J. Zhao, H. F. Jiang, B. Z. Tang, Chem. Sci. 2019, 10, 7076–7081.
- 10D. D. La, S. V. Bhosale, L. A. Jones, S. V. Bhosale, ACS Appl. Mater. Interfaces 2018, 10, 12189–12216.
- 11
- 11aG. X. Feng, B. Liu, Small 2016, 12, 6528–6535;
- 11bX. G. Gu, R. T. K. Kwok, J. W. Y. Lam, B. Z. Tang, Biomaterials 2017, 146, 115–135.
- 12J. W. Chen, B. Xu, X. Y. Ouyang, B. Z. Tang, Y. Cao, J. Phys. Chem. A 2004, 108, 7522–7526.
- 13C. J. Bhongale, C.-W. Chang, C.-S. Lee, E. W.-G. Diau, C.-S. Hsu, J. Phys. Chem. B 2005, 109, 13472–13482.
- 14K. Itami, Y. Ohashi, J. Yoshida, J. Org. Chem. 2005, 70, 2778–2792.
- 15
- 15aZ. Y. Yang, Z. G. Chi, T. Yu, X. Q. Zhang, M. N. Chen, B. J. Xu, S. W. Liu, Y. Zhang, J. R. Xu, J. Mater. Chem. 2009, 19, 5541–5546;
- 15bB. J. Xu, Z. G. Chi, Z. Y. Yang, J. B. Chen, S. Z. Deng, H. Y. Li, X. F. Li, Y. Zhang, N. S. Xu, J. R. Xu, J. Mater. Chem. 2010, 20, 4135–4141.
- 16T. Yu, D. P. Ou, L. Y. Wang, S. Z. Zheng, Z. Y. Yang, Y. Zhang, Z. G. Chi, S. W. Liu, J. R. Xu, M. P. Aldred, Mater. Chem. Front. 2017, 1, 1900–1904.
- 17
- 17aD. Liu, J. Li, J. Liu, X. Q. Lu, M. X. Hu, Y. Li, Z. B. Shu, Z. J. Ni, S. Ding, L. Jiang, Y. G. Zhen, X. T. Zhang, H. L. Dong, W. P. Hu, J. Mater. Chem. C 2018, 6, 3856–3860;
- 17bG. L. Niu, X. L. Zheng, Z. Zhao, H. K. Zhang, J. G. Wang, X. W. He, Y. C. Chen, X. J. Shi, C. Ma, R. T. K. Kwok, J. W. Y. Lam, H. H. Y. Sung, I. D. Williams, K. S. Wong, P. F. Wang, B. Z. Tang, J. Am. Chem. Soc. 2019, 141, 15111–15120.
- 18H. Lu, K. Wang, B. B. Liu, M. Wang, M. M. Huang, Y. Zhang, J. P. Yang, Mater. Chem. Front. 2019, 3, 331–338.
- 19
- 19aM. Mon, J. Ferrando-Soria, T. Grancha, F. R. Fortea-Pérez, J. Gascon, A. Leyva-Pérez, D. Armentano, E. Pardo, J. Am. Chem. Soc. 2016, 138, 7864–7867;
- 19bS. L. Lu, Y. M. Hu, S. Wan, R. McCaffrey, Y. H. Jin, H. W. Gu, W. Zhang, J. Am. Chem. Soc. 2017, 139, 17082–17088;
- 19cJ. Y. Sun, L. W. Zhang, Y. S. Hu, J. G. Fang, Sens. Actuators B 2018, 266, 447–454;
- 19dL. K. Huang, B. Chen, X. P. Zhang, C. O. Trindle, F. Liao, Y. C. Wang, H. Miao, Y. Luo, G. Q. Zhang, Angew. Chem. Int. Ed. 2018, 57, 16046–16050; Angew. Chem. 2018, 130, 16278–16282.
- 20
- 20aF. H. Xiao, H. Xie, S. W. Liu, G. J. Deng, Adv. Synth. Catal. 2014, 356, 364–368;
- 20bY.-M. Lin, G.-P. Lu, C. Cai, W.-B. Yi, Org. Lett. 2015, 17, 3310–3313;
- 20cF. H. Xiao, S. Q. Chen, J. X. Tian, H. W. Huang, Y. J. Liu, G.-J. Deng, Green Chem. 2016, 18, 1538–1546;
- 20dY.-J. Guo, S. Lu, L.-L. Tian, E.-L. Huang, X.-Q. Hao, X. J. Zhu, T. Shao, M.-P. Song, J. Org. Chem. 2018, 83, 338–349;
- 20eP. L. Bao, L. L. Wang, H. L. Yue, Y. Shao, J. W. Wen, D. S. Yang, X. H. Zhao, H. Wang, W. Wei, J. Org. Chem. 2019, 84, 2976–2983.
- 21S. W. Liu, L. C. Tang, H. Chen, F. Zhao, G. J. Deng, Org. Biomol. Chem. 2014, 12, 6076–6079.
- 22
- 22aY. L. Gao, Y. Gao, X. D. Tang, J. W. Peng, M. Hu, W. Q. Wu, H. F. Jiang, Org. Lett. 2016, 18, 1158–1161;
- 22bY. M. Lin, G. P. Lu, G. X. Wang, W. B. Yi, Adv. Synth. Catal. 2016, 358, 4100–4105.
- 23
- 23aY. C. Ding, W. Wu, W. N. Zhao, Y. W. Li, P. Xie, Y. Q. Huang, Y. Liu, A. H. Zhou, Org. Biomol. Chem. 2016, 14, 1428–1431;
- 23bW. Q. Wu, Y. N. An, J. X. Li, S. R. Yang, Z. Z. Zhu, H. F. Jiang, Org. Chem. Front. 2017, 4, 1751–1756;
- 23cY.-M. Lin, G.-P. Lu, G.-X. Wang, W.-B. Yi, J. Org. Chem. 2017, 82, 382–389;
- 23dD. Y. Wang, R. X. Zhang, W. Ning, Z. H. Yan, S. Lin, Org. Biomol. Chem. 2016, 14, 5136–5140.
- 24G. Bogonda, D. V. Patil, H. Y. Kim, K. Oh, Org. Lett. 2019, 21, 3774–3779.
- 25
- 25aJ. Shi, X.-D. Tang, Y.-C. Wu, H.-N. Li, L.-J. Song, Z.-Y. Wang, Eur. J. Org. Chem. 2015, 1193–1197;
- 25bJ. Shi, X.-D. Tang, Y.-C. Wu, J.-F. Fang, L. Cao, X.-Y. Chen, Z.-Y. Wang, RSC Adv. 2016, 6, 25651–25655;
- 25cL. Cao, J.-X. Li, H.-Q. Wu, K. Jiang, Z.-F. Hao, S.-H. Luo, Z.-Y. Wang, ACS Sustainable Chem. Eng. 2018, 6, 4147–4153;
- 25dL. Cao, S.-H. Luo, K. Jiang, Z.-F. Hao, B.-W. Wang, C.-M. Pang, Z.-Y. Wang, Org. Lett. 2018, 20, 4754–4758.
- 26CCDC 1921763 (3 a), 1921764 (3 c), 1921765 (3 g) and 1921766 (3 i) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 27S. Y. Ni, L. J. Zhang, W. Z. Zhang, H. B. Mei, J. L. Han, Y. Pan, J. Org. Chem. 2016, 81, 9470–9475.
- 28
- 28aH. Sun, X.-X. Tang, B.-X. Miao, Y. Yang, Z. H. Ni, Sens. Actuators B 2018, 267, 448–456;
- 28bC. Gao, M. K. Hossain, M. A. Wahab, J. Y. Xiong, B.-M. Qiu, H. H. Luo, W. Li, Dyes Pigm. 2019, 160, 909–914.
- 29S. Guieu, F. Cardona, J. Rocha, A. M. S. Silva, Chem. Eur. J. 2018, 24, 17262–17267.
- 30
- 30aM. K. Nayak, B.-H. Kim, J. E. Kwon, S. Park, J. Seo, J. W. Chung, S. Y. Park, Chem. Eur. J. 2010, 16, 7437–7447;
- 30bX. G. Liu, Q. L. Qiao, W. M. Tian, W. J. Liu, J. Chen, M. J. Lang, Z. C. Xu, J. Am. Chem. Soc. 2016, 138, 6960–6963;
- 30cS. Sasaki, G. P. C. Drummen, G. Konishi, J. Mater. Chem. C 2016, 4, 2731–2743;
- 30dH. Naito, K. Nishino, Y. Morisaki, K. Tanaka, Y. Chujo, Angew. Chem. Int. Ed. 2017, 56, 254–259; Angew. Chem. 2017, 129, 260–265;
- 30eH. Yan, X. L. Meng, B. Y. Li, S. S. Ge, Y. Lu, Dyes Pigm. 2017, 146, 479–490.
- 31L. Viglianti, N. L. C. Leung, N. Xie, X. G. Gu, H. H. Y. Sung, Q. Miao, I. D. Williams, E. Licandro, B. Z. Tang, Chem. Sci. 2017, 8, 2629–2639.
- 32S. Redon, G. Eucat, M. Ipuy, E. Jeanneau, I. Gautier-Luneau, A. Ibanez, C. Andraud, Y. Bretonnière, Dyes Pigm. 2018, 156, 116–132.
- 33L. R. Adil, P. Gopikrishna, P. K. Iyer, ACS Appl. Mater. Interfaces 2018, 10, 27260–27268.
- 34M. Chen, X. L. Hu, J. K. Liu, B. X. Li, N. L. C. Leung, L. Viglianti, T. S. Cheung, H. H. Y. Sung, R. T. K. Kwok, I. D. Williams, A. J. Qin, J. W. Y. Lam, B. Z. Tang, Chem. Sci. 2018, 9, 7829–7834.
- 35Z. G. Song, W. J. Zhang, M. J. Jiang, H. H. Y. Sung, R. T. K. Kwok, H. Nie, I. D. Williams, B. Liu, B. Z. Tang, Adv. Funct. Mater. 2016, 26, 824–832.
- 36X. C. Sun, Y. Wang, Y. Lei, Chem. Soc. Rev. 2015, 44, 8019–8061.
- 37W.-M. Wan, D. Tian, Y.-N. Jing, X.-Y. Zhang, W. Wu, H. Ren, H.-L. Bao, Angew. Chem. Int. Ed. 2018, 57, 15510–15516; Angew. Chem. 2018, 130, 15736–15742.
- 38H. W. Ma, F. Li, Z. X. Zhang, M. Zhang, Sens. Actuators B 2017, 244, 1080–1084.
- 39
- 39aJ.-F. Xiong, S.-H. Luo, J.-P. Huo, J.-Y. Liu, S.-X. Chen, Z.-Y. Wang, J. Org. Chem. 2014, 79, 8366–8373;
- 39bJ.-F. Xiong, J.-X. Li, G.-Z. Mo, J.-P. Huo, J.-Y. Liu, X.-Y. Chen, Z.-Y. Wang, J. Org. Chem. 2014, 79, 11619–11630;
- 39cY.-C. Wu, J.-P. Huo, L. Cao, S. Ding, L.-Y. Wang, D.-R. Cao, Z.-Y. Wang, Sens. Actuators B 2016, 237, 865–875;
- 39dY.-C. Wu, S.-H. Luo, L. Cao, K. Jiang, L.-Y. Wang, J.-C. Xie, Z.-Y. Wang, Anal. Chim. Acta 2017, 976, 74–83;
- 39eY.-C. Wu, J.-Y. You, K. Jiang, J.-C. Xie, S.-L. Li, D.-R. Cao, Z.-Y. Wang, Dyes Pigm. 2017, 140, 47–55;
- 39fY.-C. Wu, J.-Y. You, K. Jiang, H.-Q. Wu, J.-F. Xiong, Z.-Y. Wang, Dyes Pigm. 2018, 149, 1–7;
- 39gK. Jiang, Y.-C. Wu, H.-Q. Wu, S.-L. Li, S.-H. Luo, Z.-Y. Wang, J. Photochem. Photobiol. A 2018, 350, 52–58;
- 39hY.-C. Wu, K. Jiang, S.-H. Luo, L. Cao, H.-Q. Wu, Z.-Y. Wang, Spectrochim. Acta Part A 2019, 206, 632–641;
- 39iK. Jiang, S.-H. Luo, C.-M. Pang, B.-W. Wang, H.-Q. Wu, Z.-Y. Wang, Dyes Pigm. 2019, 162, 367–376;
- 39jK. Jiang, S.-H. Chen, S.-H. Luo, C.-M. Pang, X.-Y. Wu, Z.-Y. Wang, Dyes Pigm. 2019, 167, 164–173.