Construction of Quaternary Carbon Center by Catalytic Asymmetric Alkylation of 3-Arylpiperidin-2-ones Under Phase-Transfer Conditions
Tomoaki Inukai
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto, 606-8502 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Taichi Kano
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto, 606-8502 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Keiji Maruoka
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto, 606-8502 Japan
Department of Organocatalytic Chemistry, Graduate School of PharmaceuticalSciences, Kyoto University, Sakyo, Kyoto, 606-8501 Japan
Guangdong University of Technology, Guangzhou, 510006 China
School of Chemical Engineering and Light Industry, Guangdong University of Technology, Guangzhou, 510006 China
Search for more papers by this authorTomoaki Inukai
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto, 606-8502 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Taichi Kano
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto, 606-8502 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Keiji Maruoka
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto, 606-8502 Japan
Department of Organocatalytic Chemistry, Graduate School of PharmaceuticalSciences, Kyoto University, Sakyo, Kyoto, 606-8501 Japan
Guangdong University of Technology, Guangzhou, 510006 China
School of Chemical Engineering and Light Industry, Guangdong University of Technology, Guangzhou, 510006 China
Search for more papers by this authorGraphical Abstract
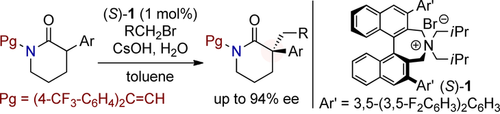
In phase: δ-Lactams having a chiral quaternary carbon center were synthesized through an asymmetric alkylation of 3-arylpiperidin-2-ones under phase-transfer conditions. A 2,2-diarylvinyl group on the δ-lactam nitrogen atom plays a crucial role as a novel protecting group and an achiral auxiliary for improving both yield and enantioselectivity of the reaction.
Abstract
A highly enantioselective synthesis of δ-lactams having a chiral quaternary carbon center at the α-position has been developed through an asymmetric alkylation of 3-arylpiperidin-2-ones under phase-transfer conditions. In this transformation, a 2,2-diarylvinyl group on the δ-lactam nitrogen atom plays a crucial role as a novel protecting group and an achiral auxiliary for improving both yield and enantioselectivity of the reaction.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201913518-sup-0001-misc_information.pdf18.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For piperidine-containing alkaloids, see:
- 1a“Pyridine and Piperidine Alkaloids: an Update”: M. J. Schneider in Alkaloids: Chemical and Biochemical Perspectives, Vol. 10 (Eds.: ), Pergamon, Oxford, 1996, p. 155;
10.1016/S0735-8210(96)80026-4 Google Scholar
- 1bD. O'Hagan, Nat. Prod. Rep. 2000, 17, 435;
- 1cJ. P. Michael, Nat. Prod. Rep. 2008, 25, 139;
- 1dE. Vitaku, D. T. Smith, J. T. Njardarson, J. Med. Chem. 2014, 57, 10257.
- 2
- 2aR. W. Hartmann, C. Batzl, T. M. Pongratz, A. Mannschreck, J. Med. Chem. 1992, 35, 2210;
- 2bG. Fogliato, G. Fronza, C. Fuganti, P. Grasselli, S. Servi, J. Org. Chem. 1995, 60, 5693.
- 3
- 3aX. Emonds-Alt, D. Bichon, J. P. Ducoux, M. Heaulme, B. Miloux, M. Poncelet, V. Proietto, D. Van Broeck, P. Vilain, G. Neliat, P. Soubrié, G. Le Fur, J. C. Brelèere, Life Sci. 1994, 56, P L27;
- 3bH. G. Chen, F.-Z. Chung, O. P. Goel, D. Johnson, S. Kesten, J. Knobelsdorf, H. T. Lee, J. R. Rubin, Bioorg. Med. Chem. Lett. 1997, 7, 555;
- 3cD. Alker, T. V. Magee, G. N. Maw, D. S. Middleton (Pfizer Inc.), Patent WO 99/1451, 1999.
- 4A. R. MacKenzie, A. P. Marchington, D. S. Middleton, S. D. Newman, B. C. Jones, J. Med. Chem. 2002, 45, 5365.
- 5For reviews on catalytic asymmetric synthesis of quaternary carbon centers, see:
- 5aE. J. Corey, A. Guzman-Perez, Angew. Chem. Int. Ed. 1998, 37, 388;
10.1002/(SICI)1521-3773(19980302)37:4<388::AID-ANIE388>3.0.CO;2-V PubMed Web of Science® Google ScholarAngew. Chem. 1998, 110, 402;
- 5bJ. Christoffers, A. Mann, Angew. Chem. Int. Ed. 2001, 40, 4591;
10.1002/1521-3773(20011217)40:24<4591::AID-ANIE4591>3.0.CO;2-V CAS PubMed Web of Science® Google ScholarAngew. Chem. 2001, 113, 4725;
- 5cI. Denissova, L. Barriault, Tetrahedron 2003, 59, 10105;
- 5dJ. Vesely, R. Rios, ChemCatChem 2012, 4, 942;
- 5eI. Marek, Y. Minko, M. Pasco, T. Mejuch, N. Gilboa, H. Chechik, J. P. Das, J. Am. Chem. Soc. 2014, 136, 2682.
- 6
- 6aC. Michon, A. Béthegnies, F. Capet, P. Roussel, A. de Filippis, D. Gomez-Pardo, J. Cossy, F. Agbossou-Niedercorn, Eur. J. Org. Chem. 2013, 4979; See also:
- 6bA. Nowicki, J. Keldenich, F. Agbossou-Niedercorn, Eur. J. Org. Chem. 2007, 6124.
- 7S. Nunokawa, M. Minamisawa, K. Nakano, Y. Ichikawa, H. Kotsuki, Synlett 2015, 26, 2301.
- 8
- 8aC. I. Jette, I. Geibel, S. Bachman, M. Hayashi, S. Sakurai, H. Shimizu, J. B. Morgan, B. M. Stoltz, Angew. Chem. Int. Ed. 2019, 58, 4297; Angew. Chem. 2019, 131, 4341; See also the related asymmetric arylation:
- 8bM. Bella, S. Kobbelgaard, K. A. Jørgensen, J. Am. Chem. Soc. 2005, 127, 3670.
- 9For representative examples, see:
- 9aU. H. Dolling, P. Davis, E. J. J. Grabowski, J. Am. Chem. Soc. 1984, 106, 446;
- 9bW. Nerinckx, M. Vandewalle, Tetrahedron: Asymmetry 1990, 1, 265;
- 9cS. Arai, M. Oku, T. Ishida, T. Shioiri, Tetrahedron Lett. 1999, 40, 6785;
- 9dT. Ooi, T. Miki, M. Taniguchi, M. Shiraishi, M. Takeuchi, K. Maruoka, Angew. Chem. Int. Ed. 2003, 42, 3796; Angew. Chem. 2003, 115, 3926;
- 9eE. J. Park, M. H. Kim, D. Y. Kim, J. Org. Chem. 2004, 69, 6897;
- 9fA. E. Nibbs, A.-L. Baize, R. M. Herter, K. A. Scheidt, Org. Lett. 2009, 11, 4010;
- 9gT. Hashimoto, K. Sakata, K. Maruoka, Angew. Chem. Int. Ed. 2009, 48, 5014; Angew. Chem. 2009, 121, 5114;
- 9hS. Hong, J. Lee, M. Kim, Y. Park, C. Park, M.-h. Kim, S.-s. Jew, H.-g. Park, J. Am. Chem. Soc. 2011, 133, 4924;
- 9iT. Kanemitsu, S. Koga, D. Nagano, M. Miyazaki, K. Nagata, T. Itoh, ACS Catal. 2011, 1, 1331;
- 9jT. Kano, Y. Hayashi, K. Maruoka, J. Am. Chem. Soc. 2013, 135, 7134;
- 9kW. Chen, W. Yang, L. Yan, C.-H. Tan, Z. Jiang, Chem. Commun. 2013, 49, 9854;
- 9lB. Xiang, K. M. Belyk, R. A. Reamer, N. Yasuda, Angew. Chem. Int. Ed. 2014, 53, 8375; Angew. Chem. 2014, 126, 8515;
- 9mB. Teng, W. Chen, S. Dong, C. W. Kee, D. A. Gandamana, L. Zong, C.-H. Tan, J. Am. Chem. Soc. 2016, 138, 9935;
- 9nR. Craig, E. Sorrentino, S. J. Connon, Chem. Eur. J. 2018, 24, 4528.
- 10For selected examples on the synthesis of δ-lactams and related compounds having a chiral quaternary carbon center at the α-position, see:
- 10aM. Amat, O. Lozano, C. Escolano, E. Molins, J. Bosch, J. Org. Chem. 2007, 72, 4431;
- 10bT. A. Moss, B. Alonso, D. R. Fenwick, D. J. Dixon, Angew. Chem. Int. Ed. 2010, 49, 568; Angew. Chem. 2010, 122, 578;
- 10cY. Park, Y. J. Lee, S. Hong, M.-h. Kim, M. Lee, T.-S. Kim, J. K. Lee, S.-s. Jew, H.-g. Park, Adv. Synth. Catal. 2011, 353, 3313;
- 10dD. C. Behenna, Y. Liu, T. Yurino, J. Kim, D. E. White, S. C. Virgil, B. M. Stoltz, Nat. Chem. 2012, 4, 130;
- 10eY. Numajiri, B. P. Pritchett, K. Chiyoda, B. M. Stoltz, J. Am. Chem. Soc. 2015, 137, 1040;
- 10fG. Pandey, J. Khamrai, A. Mishra, Org. Lett. 2018, 20, 166;
- 10gY. Lu, E. L. Goldstein, B. M. Stoltz, Org. Lett. 2018, 20, 5657;
- 10hT. Song, S. Arseniyadis, J. Cossy, Chem. Eur. J. 2018, 24, 8076;
- 10iA. Ngamnithiporn, C. I. Jette, S. Bachman, S. C. Virgil, B. M. Stoltz, Chem. Sci. 2018, 9, 2547;
- 10jB. M. Trost, Y. Bai, W.-J. Bai, J. E. Schultz, J. Am. Chem. Soc. 2019, 141, 4811.
- 11When the TBAB-catalyzed reaction of 3-phenylpiperidin-2-one 1 d with benzyl bromide was performed in the presence of KOH for 2 h at 0 °C, the benzylated product 2 d was obtained in 70 % yield. In contrast, the reaction of 3-methylpiperidin-2-one, having an N-2,2-diarylvinyl group, with benzyl bromide gave the product in 2 % yield despite the longer reaction time (12 h). This result can be explained by slow deprotonation, since use of a stronger base, CsOH increased the yield to 15 %.
- 12P. F. Keusenkothen, M. B. Smith, J. Chem. Soc. Perkin Trans. 1 1994, 2485.
- 13Hydroboration of enamines or enamides with BH3 is known, see:
- 13aB. Singaram, C. T. Goralski, G. B. Fisher, J. Org. Chem. 1991, 56, 5691;
- 13bG. A. Molander, F. Vargas, Org. Lett. 2007, 9, 203.