Intramolecular Borylation via Sequential B−Mes Bond Cleavage for the Divergent Synthesis of B,N,B-Doped Benzo[4]helicenes
Correction(s) for this article
-
Corrigendum: Intramolecular Borylation via Sequential B−Mes Bond Cleavage for the Divergent Synthesis of B,N,B-Doped Benzo[4]helicenes
- Julius A. Knöller,
- Guoyun Meng,
- Xiang Wang,
- David Hall,
- Anton Pershin,
- David Beljonne,
- Yoann Olivier,
- Sabine Laschat,
- Eli Zysman-Colman,
- Suning Wang,
- Volume 59Issue 32Angewandte Chemie International Edition
- pages: 13149-13149
- First Published online: July 31, 2020
Julius A. Knöller
Department of Chemistry, Queen's University, 90 Bader Lane, Kingston, Ontario, K7L 3N6 Canada
Institute for Organic Chemistry, Stuttgart University, Pfaffenwaldring 55, 70569 Stuttgart, Germany
Search for more papers by this authorGuoyun Meng
School of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing, China
Search for more papers by this authorDr. Xiang Wang
Department of Chemistry, Queen's University, 90 Bader Lane, Kingston, Ontario, K7L 3N6 Canada
Search for more papers by this authorDavid Hall
Organic Semiconductor Centre, EaStCHEM School of Chemistry, University of St Andrews, St Andrews, KY16 9ST UK
Laboratory for Chemistry of Novel Materials, University of Mons, 7000 Mons, Belgium
Search for more papers by this authorAnton Pershin
Laboratory for Chemistry of Novel Materials, University of Mons, 7000 Mons, Belgium
Search for more papers by this authorProf. Dr. David Beljonne
Laboratory for Chemistry of Novel Materials, University of Mons, 7000 Mons, Belgium
Search for more papers by this authorDr. Yoann Olivier
Unité de Chimie Physique Théorique et Structurale & Laboratoire de Physique du Solide, Namur Institute of Structured Matter, Université de Namur, Rue de Bruxelles, 61, 5000 Namur, Belgium
Search for more papers by this authorProf. Dr. Sabine Laschat
Institute for Organic Chemistry, Stuttgart University, Pfaffenwaldring 55, 70569 Stuttgart, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Eli Zysman-Colman
Organic Semiconductor Centre, EaStCHEM School of Chemistry, University of St Andrews, St Andrews, KY16 9ST UK
Search for more papers by this authorCorresponding Author
Prof. Dr. Suning Wang
Department of Chemistry, Queen's University, 90 Bader Lane, Kingston, Ontario, K7L 3N6 Canada
School of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing, China
Search for more papers by this authorJulius A. Knöller
Department of Chemistry, Queen's University, 90 Bader Lane, Kingston, Ontario, K7L 3N6 Canada
Institute for Organic Chemistry, Stuttgart University, Pfaffenwaldring 55, 70569 Stuttgart, Germany
Search for more papers by this authorGuoyun Meng
School of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing, China
Search for more papers by this authorDr. Xiang Wang
Department of Chemistry, Queen's University, 90 Bader Lane, Kingston, Ontario, K7L 3N6 Canada
Search for more papers by this authorDavid Hall
Organic Semiconductor Centre, EaStCHEM School of Chemistry, University of St Andrews, St Andrews, KY16 9ST UK
Laboratory for Chemistry of Novel Materials, University of Mons, 7000 Mons, Belgium
Search for more papers by this authorAnton Pershin
Laboratory for Chemistry of Novel Materials, University of Mons, 7000 Mons, Belgium
Search for more papers by this authorProf. Dr. David Beljonne
Laboratory for Chemistry of Novel Materials, University of Mons, 7000 Mons, Belgium
Search for more papers by this authorDr. Yoann Olivier
Unité de Chimie Physique Théorique et Structurale & Laboratoire de Physique du Solide, Namur Institute of Structured Matter, Université de Namur, Rue de Bruxelles, 61, 5000 Namur, Belgium
Search for more papers by this authorProf. Dr. Sabine Laschat
Institute for Organic Chemistry, Stuttgart University, Pfaffenwaldring 55, 70569 Stuttgart, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Eli Zysman-Colman
Organic Semiconductor Centre, EaStCHEM School of Chemistry, University of St Andrews, St Andrews, KY16 9ST UK
Search for more papers by this authorCorresponding Author
Prof. Dr. Suning Wang
Department of Chemistry, Queen's University, 90 Bader Lane, Kingston, Ontario, K7L 3N6 Canada
School of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing, China
Search for more papers by this authorGraphical Abstract
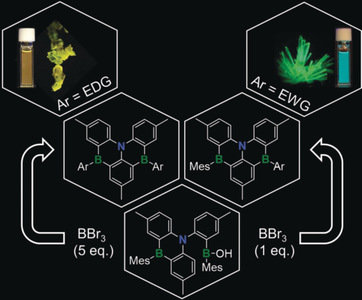
A new and divergent intramolecular borylation method has been found to be highly effective for the synthesis of a variety of symmetrically and unsymmetrically functionalized B,N,B-[4]helicenes with potential applications as thermally activated delayed fluorescence (TADF) emitters in organic light-emitting diodes (OLEDs). EDG/EWG=electron-donating/-withdrawing group, Mes=Mesityl.
Abstract
New symmetric and unsymmetric B,N,B-doped benzo[4]helicenes 3–6 a/b have been achieved in good yields, using a three-step process, starting from N(tolyl)3 in a highly divergent manner (7 examples). A borinic acid functionalized 1,4-B,N-anthracene 1 was found to display unprecedented reactivity, acting as a convenient and highly effective precursor for selective formation of bromo-substituted B,N,B-benzo[4]helicenes 2 a/2 b via intramolecular borylation and sequential B−Mes bond cleavage in the presence of BBr3. Subsequent reaction of 2 a/2 b with Ar-Li provided a highly effective toolbox for the preparation of symmetrically/unsymmetrically functionalized B,N,B-helicenes. Their high photoluminescence quantum yields along with the small ΔEST suggest their potential as thermally activated delayed fluorescence (TADF) emitters for organic light-emitting diodes (OLEDs).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201912340-sup-0001-misc_information.pdf6.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. Hirai, N. Tanaka, M. Sakai, S. Yamaguchi, Chem. Rev. 2019, 119, 8291–8331;
- 1bS. K. Mellerup, S. Wang, Trends Chem. 2019, 1, 77–89;
- 1cE. von Grotthuss, A. John, T. Kaese, M. Wagner, Asian J. Org. Chem. 2018, 7, 37–53;
- 1dL. Ji, S. Griesbeck, T. B. Marder, Chem. Sci. 2017, 8, 846–863;
- 1eA. Escande, M. J. Ingleson, Chem. Commun. 2015, 51, 6257–6274;
- 1fF. Jäkle, Chem. Rev. 2010, 110, 3985–4022.
- 2
- 2aT. L. Wu, M. J. Huang, C. C. Lin, P. Y. Huang, T. Y. Chou, R. W. Chen-Cheng, H. W. Lin, R. S. Liu, C. H. Cheng, Nat. Photonics 2018, 12, 235–240;
- 2bA. John, M. Bolte, H. Lerner, M. Wagner, Angew. Chem. Int. Ed. 2017, 56, 5588–5592; Angew. Chem. 2017, 129, 5680–5684;
- 2cL. G. Mercier, W. E. Piers, M. Parvez, Angew. Chem. Int. Ed. 2009, 48, 6108–6111; Angew. Chem. 2009, 121, 6224–6227;
- 2dT. Agou, J. Kobayashi, T. Kawashima, Chem. Commun. 2007, 3204–3206;
- 2eT. Agou, J. Kobayashi, T. Kawashima, Org. Lett. 2006, 8, 2241–2244.
- 3
- 3aY. Kondo, K. Yoshiura, S. Kitera, H. Nishi, S. Oda, H. Gotoh, Y. Sasada, M. Yanai, T. Hatakeyama, Nat. Photonics 2019, 13, 678–682;
- 3bG. Meng, X. Chen, X. Wang, N. Wang, T. Peng, S. Wang, Adv. Opt. Mater. 2019, 7, 1–14;
- 3cS. Nakatsuka, N. Yasuda, T. Hatakeyama, J. Am. Chem. Soc. 2018, 140, 13562–13565;
- 3dK. Matsui, S. Oda, K. Yoshiura, K. Nakajima, N. Yasuda, T. Hatakeyama, J. Am. Chem. Soc. 2018, 140, 1195–1198;
- 3eX. Liang, Z. P. Yan, H. B. Han, Z. G. Wu, Y. X. Zheng, H. Meng, J. L. Zuo, W. Huang, Angew. Chem. Int. Ed. 2018, 57, 11316–11320; Angew. Chem. 2018, 130, 11486–11490;
- 3fD. T. Yang, T. Nakamura, Z. He, X. Wang, A. Wakamiya, T. Peng, S. Wang, Org. Lett. 2018, 20, 6741–6745;
- 3gS. Nakatsuka, H. Gotoh, K. Kinoshita, N. Yasuda, T. Hatakeyama, Angew. Chem. Int. Ed. 2017, 56, 5087–5090; Angew. Chem. 2017, 129, 5169–5172;
- 3hT. Hatakeyama, K. Shiren, K. Nakajima, S. Nomura, S. Nakatsuka, K. Kinoshita, J. Ni, Y. Ono, T. Ikuta, Adv. Mater. 2016, 28, 2777–2781;
- 3iM. Numano, N. Nagami, S. Nakatsuka, T. Katayama, K. Nakajima, S. Tatsumi, N. Yasuda, T. Hatakeyama, Chem. Eur. J. 2016, 22, 11574–11577;
- 3jH. Hirai, K. Nakajima, S. Nakatsuka, K. Shiren, J. Ni, S. Nomura, T. Ikuta, T. Hatakeyama, Angew. Chem. Int. Ed. 2015, 54, 13581–13585; Angew. Chem. 2015, 127, 13785–13789;
- 3kM. J. Ingleson, Synlett 2012, 23, 1411–1415;
- 3lT. Hatakeyama, S. Hashimoto, S. Seki, M. Nakamura, J. Am. Chem. Soc. 2011, 133, 18614–18617.
- 4T. Kaehler, M. Bolte, H.-W. Lerner, M. Wagner, Angew. Chem. Int. Ed. 2019, 58, 11379–11384; Angew. Chem. 2019, 131, 11501–11506.
- 5
- 5aM. Farrell, C. Mu, D. Bialas, M. Rudolf, K. Menekse, A. Krause, M. Stolte, F. Würthner, J. Am. Chem. Soc. 2019, 141, 9096–9104,
- 5bJ. M. Farrell, D. Schmidt, V. Grande, F. Würthner, Angew. Chem. Int. Ed. 2017, 56, 11846–11850; Angew. Chem. 2017, 129, 12008–12012.
- 6For Yamamoto coupling refer to:
- 6aK. Schickedanz, T. Trageser, M. Bolte, H. Lerner, M. Wagner, Chem. Commun. 2015, 51, 15808–15810; for Scholl reactions refer to:
- 6bC. Dou, S. Saito, K. Matsuo, I. Hisaki, S. Yamaguchi, Angew. Chem. Int. Ed. 2012, 51, 12206–12210; Angew. Chem. 2012, 124, 12372–12376;
- 6cS. Saito, K. Matsuo, S. Yamaguchi, J. Am. Chem. Soc. 2012, 134, 9130–9133.
- 7
- 7aM. Ando, M. Sakai, N. Ando, M. Hirai, S. Yamaguchi, Org. Biomol. Chem. 2019, 17, 5500–5504;
- 7bZ. X. Giustra, S. Y. Liu, J. Am. Chem. Soc. 2018, 140, 1184–1194;
- 7cG. Bélanger-Chabot, H. Braunschweig, D. K. Roy, Eur. J. Inorg. Chem. 2017, 4353–4368;
- 7dJ. Y. Wang, J. Pei, Chin. Chem. Lett. 2016, 27, 1139–1146;
- 7eX. Liu, Y. Zhang, B. Li, L. N. Zakharov, M. Vasiliu, D. A. Dixon, S.-Y. Liu, Angew. Chem. Int. Ed. 2016, 55, 8333–8337; Angew. Chem. 2016, 128, 8473–8477;
- 7fS. Xu, F. Haeffner, B. Li, L. N. Zakharov, S.-Y. Liu, Angew. Chem. Int. Ed. 2014, 53, 6795–6799; Angew. Chem. 2014, 126, 6913–6917;
- 7gH. Braunschweig, A. Damme, J. O. C. Jimenez-Halla, B. Pfaffinger, K. Radacki, J. Wolf, Angew. Chem. Int. Ed. 2012, 51, 10034–10037; Angew. Chem. 2012, 124, 10177–10180;
- 7hT. Agou, M. Sekine, J. Kobayashi, T. Kawashima, J. Organomet. Chem. 2009, 694, 3833–3836;
- 7iT. Agou, M. Sekine, J. Kobayashi, T. Kawashima, Chem. Commun. 2009, 1894–1896.
- 8
- 8aH. Uoyama, K. Goushi, K. Shizu, H. Nomura, C. Adachi, Nature 2012, 492, 234–238;
- 8bT. T. Bui, F. Goubard, M. Ibrahim-Ouali, D. Gigmes, F. Dumur, Beilstein J. Org. Chem. 2018, 14, 282–308;
- 8cY. Liu, C. Li, Z. Ren, S. Yan, M. R. Bryce, Nat. Rev. Mater. 2018, 3, 18020;
- 8dM. Y. Wong, E. Zysman-Colman, Adv. Mater. 2017, 29, 160544.
- 9A. W. Baggett, S. Y. Liu, J. Am. Chem. Soc. 2017, 139, 15259–15264.
- 10S. Oda, B. Kawakami, R. Kawasumi, R. Okita, T. Hatakeyama, Org. Lett. 2019, 21, 9311–9314.
- 11
- 11aY. Shen, C. F. Chen, Chem. Rev. 2012, 112, 1463–1535;
- 11bR. H. Martin, Angew. Chem. Int. Ed. Engl. 1974, 13, 649–660; Angew. Chem. 1974, 86, 727–738;
- 11cV. Berezhnaia, M. Roy, N. Vanthuyne, M. Villa, J. V. Naubron, J. Rodriguez, Y. Coquerel, M. Gingras, J. Am. Chem. Soc. 2017, 139, 18508–18511;
- 11dF. L. Hirshfeld, S. Sandler, G. M. J. Schmidt, J. Chem. Soc. 1963, 2108–2125.
- 12
- 12aX. Yin, J. Chen, R. A. Lalancette, T. B. Marder, F. Jäkle, Angew. Chem. Int. Ed. 2014, 53, 9761–9765; Angew. Chem. 2014, 126, 9919–9923;
- 12bZ. Zhang, R. M. Edkins, M. Haehnel, M. Wehner, M. Meier, J. Brand, H. Braunschweig, T. B. Marder, Chem. Sci. 2015, 6, 5922–5927.
- 13A. Pershin, D. Hall, V. Lemaur, J. C. Sancho-Garcia, L. Muccioli, E. Zysman-Colman, D. Beljonne, Y. Olivier, Nat. Commun. 2019, 10, 1–5.