A Class of FeIII Macrocyclic Complexes with Alcohol Donor Groups as Effective T1 MRI Contrast Agents
Eric M. Snyder
Department of Chemistry, University at Buffalo, State University of New York, Amherst, New York, 14260 USA
Search for more papers by this authorDidar Asik
Department of Chemistry, University at Buffalo, State University of New York, Amherst, New York, 14260 USA
Search for more papers by this authorSamira M. Abozeid
Department of Chemistry, University at Buffalo, State University of New York, Amherst, New York, 14260 USA
Search for more papers by this authorAriel Burgio
Department of Chemistry, University at Buffalo, State University of New York, Amherst, New York, 14260 USA
Search for more papers by this authorGage Bateman
Department of Chemistry, University at Buffalo, State University of New York, Amherst, New York, 14260 USA
Search for more papers by this authorSteven G. Turowski
Department of Cell Stress Biology, Roswell Park Comprehensive Cancer Center, Buffalo, New York, 14263 USA
Search for more papers by this authorDr. Joseph A. Spernyak
Department of Cell Stress Biology, Roswell Park Comprehensive Cancer Center, Buffalo, New York, 14263 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Janet R. Morrow
Department of Chemistry, University at Buffalo, State University of New York, Amherst, New York, 14260 USA
Search for more papers by this authorEric M. Snyder
Department of Chemistry, University at Buffalo, State University of New York, Amherst, New York, 14260 USA
Search for more papers by this authorDidar Asik
Department of Chemistry, University at Buffalo, State University of New York, Amherst, New York, 14260 USA
Search for more papers by this authorSamira M. Abozeid
Department of Chemistry, University at Buffalo, State University of New York, Amherst, New York, 14260 USA
Search for more papers by this authorAriel Burgio
Department of Chemistry, University at Buffalo, State University of New York, Amherst, New York, 14260 USA
Search for more papers by this authorGage Bateman
Department of Chemistry, University at Buffalo, State University of New York, Amherst, New York, 14260 USA
Search for more papers by this authorSteven G. Turowski
Department of Cell Stress Biology, Roswell Park Comprehensive Cancer Center, Buffalo, New York, 14263 USA
Search for more papers by this authorDr. Joseph A. Spernyak
Department of Cell Stress Biology, Roswell Park Comprehensive Cancer Center, Buffalo, New York, 14263 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Janet R. Morrow
Department of Chemistry, University at Buffalo, State University of New York, Amherst, New York, 14260 USA
Search for more papers by this authorGraphical Abstract
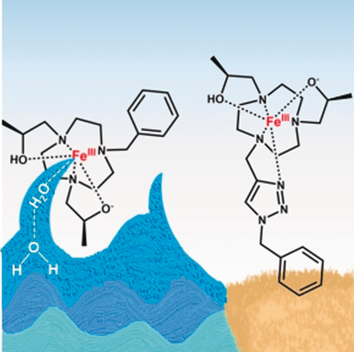
High-spin iron(III) complexes with an open coordination site for solvent interactions are effective T1 MRI contrast agents in phantoms and in live mice. A coordinated water ligand and the deprotonation of a pendant alcohol stabilize the trivalent iron center to produce strong second-sphere water interactions.
Abstract
Early studies suggested that FeIII complexes cannot compete with GdIII complexes as T1 MRI contrast agents. Now it is shown that one member of a class of high-spin macrocyclic FeIII complexes produces more intense contrast in mice kidneys and liver at 30 minutes post-injection than does a commercially used GdIII agent and also produces similar T1 relaxivity in serum phantoms at 4.7 T and 37 °C. Comparison of four different FeIII macrocyclic complexes elucidates the factors that contribute to relaxivity in vivo including solution speciation. Variable-temperature 17O NMR studies suggest that none of the complexes has a single, integral inner-sphere water that exchanges rapidly on the NMR timescale. MRI studies in mice show large in vivo differences of three of the FeIII complexes that correspond, in part, to their r1 relaxivity in phantoms. Changes in overall charge of the complex modulate contrast enhancement, especially of the kidneys.
Conflict of interest
J.R.M. is a co-founder of a company for iron contrast agents.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201912273-sup-0001-misc_information.pdf3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aR. B. Lauffer, Chem. Rev. 1987, 87, 901–927;
- 1bM. F. Tweedle, G. T. Gaughan, J. Hagan, P. W. Wedeking, P. Sibley, L. J. Wilson, D. W. Lee, Int. J. Radiat. Appl. Instrum. Part B 1988, 15, 31–36.
- 2
- 2aP. Caravan, J. J. Ellison, T. J. McMurry, R. B. Lauffer, Chem. Rev. 1999, 99, 2293–2352;
- 2bS.-P. Lin, J. J. Brown, J. Magn. Reson. Imaging 2007, 25, 884–899;
- 2cJ. Wahsner, E. M. Gale, A. Rodríguez-Rodríguez, P. Caravan, Chem. Rev. 2019, 119, 957–1057.
- 3R. Bruce, A. L. Wentland, A. K. Haemel, R. W. Garrett, D. R. Sadowski, A. Djamali, E. A. Sadowski, Invest. Radiol. 2016, 51, 701–705.
- 4
- 4aE. Gianolio, E. D. Gregorio, S. Aime, Eur. J. Inorg. Chem. 2019, 137–151;
- 4bM. Le Fur, P. Caravan, Metallomics 2019, 11, 240–254;
- 4cC. Olchowy, K. Cebulski, M. Łasecki, R. Chaber, A. Olchowy, K. Kałwak, U. Zaleska-Dorobisz, PLoS One 2017, 12, e0171704;
- 4dE. Kanal, Magn. Reson. Imaging 2016, 34, 1341–1345;
- 4eA. Radbruch, L. D. Weberling, P. J. Kieslich, J. Hepp, P. Kickingereder, W. Wick, H.-P. Schlemmer, M. Bendszus, Invest. Radiol. 2016, 51, 683–690;
- 4fN. Murata, L. F. Gonzalez-Cuyar, K. Murata, C. Fligner, R. Dills, D. Hippe, K. R. Maravilla, Invest. Radiol. 2016, 51, 447–453;
- 4gR. C. Semelka, J. Ramalho, A. Vakharia, M. AlObaidy, L. M. Burke, M. Jay, M. Ramalho, Magn. Reson. Imaging 2016, 34, 1383–1390.
- 5
- 5aD. Pan, A. H. Schmieder, S. A. Wickline, G. M. Lanza, Tetrahedron 2011, 67, 8431–8444;
- 5bB. Drahoš, I. Lukeš, É. Tóth, Eur. J. Inorg. Chem. 2012, 1975–1986;
- 5cM. Botta, F. Carniato, D. Esteban-Gómez, C. Platas-Iglesias, L. Tei, Future Med. Chem. 2019, 11, 1461–1483.
- 6G. B. Toth, C. G. Varallyay, A. Horvath, M. R. Bashir, P. L. Choyke, H. E. Daldrup-Link, E. Dosa, J. P. Finn, S. Gahramanov, M. Harisinghani, I. Macdougall, A. Neuwelt, S. S. Vasanawala, P. Ambady, R. Barajas, J. S. Cetas, J. Ciporen, T. J. DeLoughery, N. D. Doolittle, R. Fu, J. Grinstead, A. R. Guimaraes, B. E. Hamilton, X. Li, H. L. McConnell, L. L. Muldoon, G. Nesbit, J. P. Netto, D. Petterson, W. D. Rooney, D. Schwartz, L. Szidonya, E. A. Neuwelt, Kidney Int. 2017, 92, 47–66.
- 7
- 7aR. J. Motekaitis, A. E. Martell, M. J. Welch, Inorg. Chem. 1990, 29, 1463–1467;
- 7bC. J. Bannochie, A. E. Martell, Inorg. Chem. 1991, 30, 1385–1392.
- 8N. Kuźnik, M. Wyskocka, Eur. J. Inorg. Chem. 2016, 445–458.
- 9
- 9aH. Wang, V. C. Jordan, I. A. Ramsay, M. Sojoodi, B. C. Fuchs, K. K. Tanabe, P. Caravan, E. M. Gale, J. Am. Chem. Soc. 2019, 141, 5916–5925;
- 9bP. Boehm-Sturm, A. Haeckel, R. Hauptmann, S. Mueller, C. K. Kuhl, E. A. Schellenberger, Radiology 2018, 286, 537–546;
- 9cM. F. Tweedle, Radiology 2018, 286, 409–411.
- 10
- 10aR. K. Khan, S. P. Gadiraju, M. Kumar, G. A. Hatmaker, B. J. Fisher, R. Natarajan, J. E. Reiner, M. M. Collinson, ACS Sens. 2018, 3, 1601–1608;
- 10bD. J. Kosman, Metallomics 2018, 10, 370–377.
- 11
- 11aP. Caravan, Chem. Soc. Rev. 2006, 35, 512–523;
- 11bM. Botta, Eur. J. Inorg. Chem. 2000, 399–407.
10.1002/(SICI)1099-0682(200003)2000:3<399::AID-EJIC399>3.0.CO;2-B CAS Web of Science® Google Scholar
- 12E. Boros, E. M. Gale, P. Caravan, Dalton Trans. 2015, 44, 4804–4818.
- 13R. Pujales-Paradela, M. Regueiro-Figueroa, D. Esteban-Gómez, C. Platas-Iglesias, Contrast Agents for MRI, Eds.: , Royal Society of Chemistry, Croydon, UK, 2018, pp. 448–478.
- 14A. E. Martell, R. J. Motekaitis, M. J. Welch, J. Chem. Soc. Chem. Commun. 1990, 1748–1749.
- 15
- 15aG. J. Stasiuk, M. P. Lowe, Dalton Trans. 2009, 9725–9727;
- 15bM. Jauregui, W. S. Perry, C. Allain, L. R. Vidler, M. C. Willis, A. M. Kenwright, J. S. Snaith, G. J. Stasiuk, M. P. Lowe, S. Faulkner, Dalton Trans. 2009, 6283–6285.
- 16
- 16aJ. C. Joyner, J. Reichfield, J. A. Cowan, J. Am. Chem. Soc. 2011, 133, 15613–15626;
- 16bK. Amreen, A. S. Kumar, Analyst 2016, 141, 2145–2149.
- 17W. H. Koppenol, R. H. Hider, Free Radical Biol. Med. 2019, 133, 3–10.
- 18R. Luckay, R. D. Hancock, I. Cukrowski, J. H. Reibenspies, Inorg. Chim. Acta 1996, 246, 159–169.
- 19A. Brausam, J. Maigut, R. Meier, P. Á. Szilágyi, H.-J. Buschmann, W. Massa, Z. Homonnay, R. van Eldik, Inorg. Chem. 2009, 48, 7864–7884.
- 20E. M. Gale, J. Zhu, P. Caravan, J. Am. Chem. Soc. 2013, 135, 18600–18608.
- 21A. Patel, D. Asik, J. A. Spernyak, P. J. Cullen, J. R. Morrow, J. Inorg. Biochem. 2019, 201, 110832.
- 22S. H. Koenig, R. D. Brown III, T. R. Lindstrom, Biophys. J. 1981, 34, 397–408.
- 23J. Wang, H. Wang, I. A. Ramsay, D. J. Erstad, B. C. Fuchs, K. K. Tanabe, P. Caravan, E. M. Gale, J. Med. Chem. 2018, 61, 8811–8824.
- 24
- 24aH. Koepsell, A. Busch, V. Gorboulev, P. Arndt, Physiology 1998, 13, 11–16;
- 24bH. Koepsell, Expert Opin. Drug Metab. Toxicol. 2015, 11, 1619–1633.
- 25P. B. Tsitovich, F. Gendron, A. Y. Nazarenko, B. N. Livesay, A. P. Lopez, M. P. Shores, J. Autschbach, J. R. Morrow, Inorg. Chem. 2018, 57, 8364–8374.
- 26
- 26aS. J. Dorazio, P. B. Tsitovich, S. A. Gardina, J. R. Morrow, J. Inorg. Biochem. 2012, 117, 212–219;
- 26bS. M. Abozeid, E. M. Snyder, T. Y. Tittiris, C. M. Steuerwald, A. Y. Nazarenko, J. R. Morrow, Inorg. Chem. 2018, 57, 2085–2095.
- 27CCDC 1952732 contains the supplementary crystallographic data for this paper. These data are provided free of charge by The Cambridge Crystallographic Data Centre.