Separation of Bromoalkanes Isomers by Nonporous Adaptive Crystals of Leaning Pillar[6]arene
Jia-Rui Wu
State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, International Joint Research Laboratory of Nano-Micro Architecture Chemistry (NMAC), College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun, 130012 P. R. China
Search for more papers by this authorProf. Bao Li
State Key Laboratory of Supramolecular Structure and Materials, Institute of Theoretical Chemistry, Jilin University, Changchun, 130012 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Ying-Wei Yang
State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, International Joint Research Laboratory of Nano-Micro Architecture Chemistry (NMAC), College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun, 130012 P. R. China
Search for more papers by this authorJia-Rui Wu
State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, International Joint Research Laboratory of Nano-Micro Architecture Chemistry (NMAC), College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun, 130012 P. R. China
Search for more papers by this authorProf. Bao Li
State Key Laboratory of Supramolecular Structure and Materials, Institute of Theoretical Chemistry, Jilin University, Changchun, 130012 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Ying-Wei Yang
State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, International Joint Research Laboratory of Nano-Micro Architecture Chemistry (NMAC), College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun, 130012 P. R. China
Search for more papers by this authorGraphical Abstract
Abstract
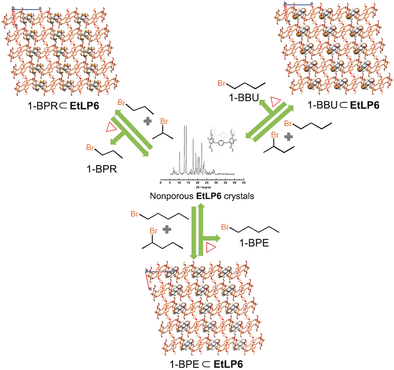
Haloalkanes are important chemicals in synthetic chemistry and petrochemical industry, but the separation of their isomers is a big hurdle. Herein, we report a facile energy-efficient adsorptive separation strategy using a new class of nonporous adaptive crystals based on leaning pillar[6]arene. Desolvated perethylated leaning pillar[6]arene crystals (EtLP6) with interesting nonporous character show a preference for 1-bromoalkane isomers over 2-bromoalkane isomers. EtLP6 is capable of separating 1-bromopropane, 1-bromobutane, and 1-bromopentane from the corresponding 1:1 (v/v) mixtures of 1/2-positional isomers with purities from 89.6 % to 96.3 % in only one adsorption cycle. The selectivity is endowed by the different host–guest binding modes and different stabilities of EtLP6 crystalloids loaded with 1- and 2-positional isomers. Significantly, the guest–adsorbed assemblies are highly stable at room temperature and EtLP6 can be reused many times without any decrease in performance.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201911965-sup-0001-misc_information.pdf5.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aP. Comba, S. Wunderlich, Chem. Eur. J. 2010, 16, 7293–7299;
- 1bL. Vasáros, H. J. Machulla, W. Tornau, J. Chromatogr. A 1971, 62, 458–461;
- 1cK. Jie, Y. Zhou, E. Li, R. Zhao, M. Liu, F. Huang, J. Am. Chem. Soc. 2018, 140, 3190–3193.
- 2M. Hartmann, S. Kunz, D. Himsl, O. Tangermann, S. Ernst, A. Wagener, Langmuir 2008, 24, 8634–8642.
- 3
- 3aZ. Bao, G. Chang, H. Xing, R. Krishna, Q. Ren, B. Chen, Energy Environ. Sci. 2016, 9, 3612–3641;
- 3bJ.-R. Li, R. J. Kuppler, H.-C. Zhou, Chem. Soc. Rev. 2009, 38, 1477–1504.
- 4
- 4aY. Peng, Y. Li, Y. Ban, H. Jin, W. Jiao, X. Liu, W. Yang, Science 2014, 346, 1356;
- 4bM. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. Keeffe, O. M. Yaghi, Science 2002, 295, 469.
- 5
- 5aD. Wu, F. Xu, B. Sun, R. Fu, H. He, K. Matyjaszewski, Chem. Rev. 2012, 112, 3959–4015;
- 5bS. Das, P. Heasman, T. Ben, S. Qiu, Chem. Rev. 2017, 117, 1515–1563.
- 6
- 6aA. P. Côté, A. I. Benin, N. W. Ockwig, M. Keeffe, A. J. Matzger, O. M. Yaghi, Science 2005, 310, 1166;
- 6bX. Feng, X. Ding, D. Jiang, Chem. Soc. Rev. 2012, 41, 6010–6022;
- 6cS.-Y. Ding, W. Wang, Chem. Soc. Rev. 2013, 42, 548–568.
- 7
- 7aL.-L. Tan, H. Li, Y. Tao, S. X.-A. Zhang, B. Wang, Y.-W. Yang, Adv. Mater. 2014, 26, 7027–7031;
- 7bN. Song, T. Kakuta, T.-a. Yamagishi, Y.-W. Yang, T. Ogoshi, Chem 2018, 4, 2029–2053;
- 7cX. Li, Z. Li, Y.-W. Yang, Adv. Mater. 2018, 30, 1800177;
- 7dT. Ogoshi, R. Sueto, M. Yagyu, R. Kojima, T. Kakuta, T.-a. Yamagishi, K. Doitomi, A. K. Tummanapelli, H. Hirao, Y. Sakata, S. Akine, M. Mizuno, Nat. Commun. 2019, 10, 479.
- 8
- 8aM. R. Anderson, B. R. Mattes, H. Reiss, R. B. Kaner, Science 1991, 252, 1412;
- 8bM. E. Davis, Nature 2002, 417, 813–821;
- 8cT. Tozawa, J. T. A. Jones, S. I. Swamy, S. Jiang, D. J. Adams, S. Shakespeare, R. Clowes, D. Bradshaw, T. Hasell, S. Y. Chong, C. Tang, S. Thompson, J. Parker, A. Trewin, J. Bacsa, A. M. Z. Slawin, A. Steiner, A. I. Cooper, Nat. Mater. 2009, 8, 973;
- 8dJ. R. Holst, A. Trewin, A. I. Cooper, Nat. Chem. 2010, 2, 915;
- 8eM. Yu, R. D. Noble, J. L. Falconer, Acc. Chem. Res. 2011, 44, 1196–1206;
- 8fJ. W. Colson, W. R. Dichtel, Nat. Chem. 2013, 5, 453.
- 9
- 9aK. Jie, Y. Zhou, E. Li, F. Huang, Acc. Chem. Res. 2018, 51, 2064–2072;
- 9bK. Jie, M. Liu, Y. Zhou, M. A. Little, S. Bonakala, S. Y. Chong, A. Stephenson, L. Chen, F. Huang, A. I. Cooper, J. Am. Chem. Soc. 2017, 139, 2908–2911;
- 9cK. Jie, M. Liu, Y. Zhou, M. A. Little, A. Pulido, S. Y. Chong, A. Stephenson, A. R. Hughes, F. Sakakibara, T. Ogoshi, F. Blanc, G. M. Day, F. Huang, A. I. Cooper, J. Am. Chem. Soc. 2018, 140, 6921–6930;
- 9dY. Zhou, K. Jie, R. Zhao, F. Huang, J. Am. Chem. Soc. 2019, 141, 11847–11851.
- 10
- 10aT. Ogoshi, R. Sueto, K. Yoshikoshi, Y. Sakata, S. Akine, T.-a. Yamagishi, Angew. Chem. Int. Ed. 2015, 54, 9849–9852; Angew. Chem. 2015, 127, 9987–9990;
- 10bT. Ogoshi, Y. Shimada, Y. Sakata, S. Akine, T.-a. Yamagishi, J. Am. Chem. Soc. 2017, 139, 5664–5667;
- 10cT. Ogoshi, K. Saito, R. Sueto, R. Kojima, Y. Hamada, S. Akine, A. M. P. Moeljadi, H. Hirao, T. Kakuta, T.-a. Yamagishi, Angew. Chem. Int. Ed. 2018, 57, 1592–1595; Angew. Chem. 2018, 130, 1608–1611.
- 11
- 11aK. Jie, Y. Zhou, E. Li, Z. Li, R. Zhao, F. Huang, J. Am. Chem. Soc. 2017, 139, 15320–15323;
- 11bK. Jie, Y. Zhou, E. Li, R. Zhao, F. Huang, Angew. Chem. Int. Ed. 2018, 57, 12845–12849; Angew. Chem. 2018, 130, 13027–13031;
- 11cE. Li, K. Jie, Y. Zhou, R. Zhao, F. Huang, J. Am. Chem. Soc. 2018, 140, 15070–15079;
- 11dE. Li, K. Jie, Y. Zhou, R. Zhao, B. Zhang, Q. Wang, J. Liu, F. Huang, ACS Appl. Mater. Interfaces 2018, 10, 23147–23153;
- 11eJ.-R. Wu, B. Li, J.-W. Zhang, Y.-W. Yang, ACS Appl. Mater. Interfaces 2019, 11, 998–1003;
- 11fE. Li, Y. Zhou, R. Zhao, K. Jie, F. Huang, Angew. Chem. Int. Ed. 2019, 58, 3981–3985; Angew. Chem. 2019, 131, 4021–4025.
- 12
- 12aJ.-R. Wu, A. U. Mu, B. Li, C.-Y. Wang, L. Fang, Y.-W. Yang, Angew. Chem. Int. Ed. 2018, 57, 9853–9858; Angew. Chem. 2018, 130, 10001–10006;
- 12bT. M. Swager, Y. Kim, Synfacts 2018, 14, 0815;
10.1055/s-0037-1610512 Google Scholar
- 12cD. Shetty, A. Trabolsi, Sci. China Chem. 2019, 62, 289;
- 12dJ.-R. Wu, Y.-W. Yang, Chem. Commun. 2019, 55, 1533–1543;
- 12eD. Dai, Z. Li, J. Yang, C. Wang, J.-R. Wu, Y. Wang, D. Zhang, Y.-W. Yang, J. Am. Chem. Soc. 2019, 141, 4756–4763;
- 12fX. Wang, J.-R. Wu, F. Liang, Y.-W. Yang, Org. Lett. 2019, 21, 5215–5218;
- 12gJ.-R. Wu, Y.-W. Yang, J. Am. Chem. Soc. 2019, 141, 12280–12287;
- 12hX. Li, J. Han, J. Qin, M. Sun, J. Wu, L. Lei, L. Fang, Y.-W. Yang, Chem. Commun. 2019, 55, 14099–14102;
- 12iZ. Liu, J. Wu, C. Wang, J. Yang, Y. Wang, Y.-W. Yang, Chin. Chem. Lett. 2019, https://doi.org/10.1016/j.cclet.2019.10.023.
- 13CCDC 1937374, 1937375, 1937376, 1937377, and 1937378 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.