6-Methylenebicyclo[3.2.1]oct-1-en-3-one: A Twisted Olefin as Diels–Alder Dienophile for Expedited Syntheses of Four Kaurane Diterpenoids
Junjie Wang
State Key Laboratory of Bioorganic & Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Dawei Ma
State Key Laboratory of Bioorganic & Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorJunjie Wang
State Key Laboratory of Bioorganic & Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Dawei Ma
State Key Laboratory of Bioorganic & Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorGraphical Abstract
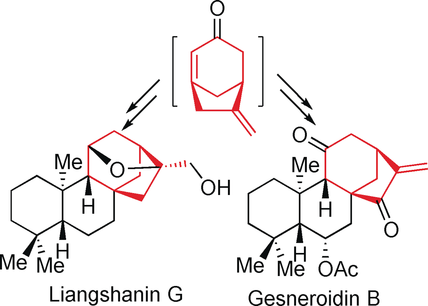
With a twist: 6-Methylenebicyclo[3.2.1]oct-1-en-3-one, a twisted and highly reactive enone, was prepared for the first time by elimination of its bromide precursor. Its reactions as a dienophile with several dienes in Diels–Alder reactions proceeded smoothly to provide tricyclic and tetracyclic adducts, which allowed short syntheses (10–11 steps) of four kurane diterpenoids including 11β-hydroxy-16-kaurene, 11α-hydroxy-16-kaurene, liangshanin G, and gesneroidin B.
Abstract
6-Methylenebicyclo[3.2.1]oct-1-en-3-one, a twisted and highly reactive enone, was prepared for the first time by elimination of its bromide precursor. Its reactions as a dienophile with several dienes in Diels–Alder reactions proceeded smoothly to provide tricyclic and tetracyclic adducts, which allowed short syntheses (10–11 steps) of four kurane diterpenoids including 11β-hydroxy-16-kaurene, 11α-hydroxy-16-kaurene, liangshanin G, and gesneroidin B.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201909349-sup-0001-misc_information.pdf3.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM.-H. Filippini, J. Rodriguez, Chem. Rev. 1999, 99, 27;
- 1bM. Presset, Y. Coquerel, J. Rodriguez, Chem. Rev. 2013, 113, 525.
- 2J. Wang, S. M. Soisson, K. Young, W. Shoop, S. Kodali, A. Galgoci, R. Painter, G. Parthasarathy, Y. S. Tang, R. Cummings, S. Ha, K. Dorso, M. Motyl, H. Jayasuriya, J. Ondeyka, K. Herath, C. W. Zhang, L. Hernandez, J. Allocco, A. Basilio, J. R. Tormo, O. Genilloud, L. Colwell, S. H. Lee, B. Michael, T. Felcetto, C. Gill, L. L. Silver, J. D. Hermes, J. W. Becker, D. Cully, S. B. Singh, Nature 2006, 441, 358.
- 3H.-D. Sun, Y.-L. Xu, F.-L. Zhang, Phytochemistry 1989, 28, 1671.
- 4H.-D. Sun, Z.-W. Lin, F.-D. Niu, Q.-T. Zhen, B. Wu, L.-Z. Lin, Phytochemistry 1995, 40, 1461.
- 5A. Furusaki, N. Hamanaka, H. Miyakoshi, T. Okuno, T. Matsumoto, Chem. Lett. 1972, 1, 783.
- 6L.-Q. Wang, S.-N. Chen, K. F. Cheng, C.-J. Li, G.-W. Qin, Phytochemistry 2000, 54, 847.
- 7M. Furber, P. Kraft-Klaunzer, L. N. Mander, M. Pour, T. Yamauchi, N. Murofushi, H. Yamane, H. Schraudolf, Aust. J. Chem. 1995, 48, 427.
- 8For reviews, see:
- 8aK. E. Lazarski, B. J. Moritz, R. J. Thomson, Angew. Chem. Int. Ed. 2014, 53, 10588; Angew. Chem. 2014, 126, 10762;
- 8bL. Zhu, S.-H. Huang, J. Yu, R. Hong, Tetrahedron Lett. 2015, 56, 23;
- 8cM.-J. Du, X.-G. Lei, Youji Huaxue 2015, 35, 2447;
- 8dY.-S. Yao, Z.-J. Yao, Chin. J. Org. Chem. 2008, 28, 1553;
- 8eL. N. Mander, Nat. Prod. Rep. 1988, 5, 541;
- 8fL. N. Mander, Nat. Prod. Rep. 2003, 20, 49;
- 8gM. Vandewalle, P. De Clercq, Tetrahedron 1985, 41, 1765.
- 9For selected references on synthetic studies toward ent-kauranoids, see:
- 9aR. A. Bell, R. E. Ireland, R. A. Partyka, J. Org. Chem. 1962, 27, 3741;
- 9bS. Masamune, J. Am. Chem. Soc. 1964, 86, 288;
- 9cR. E. Ireland, L. N. Mander, Tetrahedron Lett. 1965, 6, 2627;
10.1016/S0040-4039(00)90219-3 Google Scholar
- 9dR. B. Turner, K. H. Ganshirt, P. E. Shaw, J. D. Tauber, J. Am. Chem. Soc. 1966, 88, 1776;
- 9eK. Mori, M. Matsui, Tetrahedron 1966, 22, 879;
- 9fK. Mori, Y. Nakahara, M. Matsui, Tetrahedron Lett. 1970, 11, 2411;
10.1016/S0040-4039(01)98242-5 Google Scholar
- 9gY. Nakahara, K. Mori, M. Matsui, Agric. Biol. Chem. 1971, 35, 918;
- 9hK. Mori, Y. Nakahara, M. Matsui, Tetrahedron 1972, 28, 3217;
- 9iD. K. M. Duc, M. Fetizon, S. Lazare, J. Chem. Soc. Chem. Commun. 1975, 282;
10.1039/c39750000282 Google Scholar
- 9jF. E. Ziegler, J. A. Kloek, Tetrahedron 1977, 33, 373;
- 9kE. J. Corey, G. Wess, Y. B. Xiang, A. K. Singh, J. Am. Chem. Soc. 1987, 109, 4717;
- 9lA. K. Singh, R. K. Bakshi, E. J. Corey, J. Am. Chem. Soc. 1987, 109, 6187;
- 9mD. Backhaus, L. A. Paquette, Tetrahedron Lett. 1997, 38, 29;
- 9nE. J. Corey, K. Liu, J. Am. Chem. Soc. 1997, 119, 9929;
- 9oE. C. Cherney, J. C. Green, P. S. Baran, Angew. Chem. Int. Ed. 2013, 52, 9019; Angew. Chem. 2013, 125, 9189;
- 9pJ. T. S. Yeoman, V. W. Mak, S. E. Reisman, J. Am. Chem. Soc. 2013, 135, 11764;
- 9qJ. T. S. Yeoman, J. Y. Cha, V. W. Mak, S. E. Reisman, Tetrahedron 2014, 70, 4070;
- 9rL. Zhu, J. Luo, R. Hong, Org. Lett. 2014, 16, 2162;
- 9sC. He, J. Hu, Y. Wu, H. Ding, J. Am. Chem. Soc. 2017, 139, 6098;
- 9tW. Liu, H. Li, P.-J. Cai, Z. Wang, Z.-X. Yu, X. Lei, Angew. Chem. Int. Ed. 2016, 55, 3112; Angew. Chem. 2016, 128, 3164;
- 9uX. Zhao, W. Li, J. Wang, D. Ma, J. Am. Chem. Soc. 2017, 139, 2932;
- 9vF. Su, Y. Lu, L. Kong, J. Liu, T. Luo, Angew. Chem. Int. Ed. 2018, 57, 760; Angew. Chem. 2018, 130, 768;
- 9wL. Zhu, W. Ma, M. Zhang, M. M.-L. Lee, W.-Y. Wong, B. D. Chan, Q. Yang, W.-T. Wong, W. C.-S. Tai, C.-S. Lee, Nat. Commun. 2018, 9, 1283;
- 9xB.-K. Hong, W.-L. Liu, J. Wang, J.-B. Wu, Y. Kadonaga, P.-J. Cai, H.-X. Lou, Z.-X. Yu, H.-H. Li, X. Lei, Chem 2019, 5, 1368.
- 10For selected references, see:
- 10aE. J. Corey, R. L. Danheiser, S. Chandrasekaran, P. Siret, G. E. Keck, J.-L. Gras, J. Am. Chem. Soc. 1978, 100, 8031;
- 10bJ. M. Hook, L. N. Mander, R. Urech, J. Am. Chem. Soc. 1980, 102, 6628;
- 10cW. M. Grootaert, P. J. De Clercq, Tetrahedron Lett. 1986, 27, 1731;
- 10dH. Nagaoka, M. Shimano, Y. Yamada, Tetrahedron Lett. 1989, 30, 971;
- 10eW. Nagata, T. Wakabayashi, M. Narisada, Y. Hayase, S. Kumata, J. Am. Chem. Soc. 1971, 93, 5740;
- 10fL. Lombardo, L. N. Mander, J. Org. Chem. 1983, 48, 2298;
- 10gG. R. King, L. N. Mander, N. J. T. Monck, J. C. Morris, H. Zhang, J. Am. Chem. Soc. 1997, 119, 3828.
- 11For selected references on synthesis of grayanoids, see:
- 11aN. Hamanaka, T. Matsumoto, Tetrahedron Lett. 1972, 13, 3087;
10.1016/S0040-4039(01)85015-2 Google Scholar
- 11bS. Gasa, N. Hamanaka, S. Matsunaga, T. Okuno, N. Takeda, T. Matsumoto, Tetrahedron Lett. 1976, 17, 553;
10.1016/S0040-4039(00)77908-1 Google Scholar
- 11cT. Kan, S. Hosokawa, S. Nara, M. Oikawa, S. Ito, F. Matsuda, H. Shirahama, J. Org. Chem. 1994, 59, 5532;
- 11dA. Turlik, Y. Chen, A. C. Scruse, T. R. Newhouse, J. Am. Chem. Soc. 2019, 141, 8088;
- 11eK. Yu, Z.-N. Yang, C.-H. Liu, S.-Q. Wu, X. Hong, X.-Li. Zhao, H. Ding, Angew. Chem. Int. Ed. 2019, 58, 8556; Angew. Chem. 2019, 131, 8644.
- 12
- 12aG. A. Kraus, Y.-S. Hon, J. Sy, J. Raggon, J. Org. Chem. 1988, 53, 1397;
- 12bP.-Q. Huang, W.-S. Zhou, Tetmhedron: Asymmetry 1991, 2, 875.
- 13For reviews on bridgehead enones, see:
- 13aK. J. Shea, Tetrahedron 1980, 36, 1683;
- 13bP. M. Warner, Chem. Rev. 1989, 89, 1067;
- 13cG. A. Kraus, Y.-S. Hon, P. J. Thomas, S. Laramay, S. Liras, J. Hanson, Chem. Rev. 1989, 89, 1591.
- 14For studies on [3.3.1]bridgehead enones, see:
- 14aH. O. House, W. A. Kleschick, E. J. Zaiko, J. Org. Chem. 1978, 43, 3653;
- 14bH. O. House, M. B. DeTar, D. Van Derveer, J. Org. Chem. 1979, 44, 3793;
- 14cH. O. House, M. B. DeTar, R. F. Sieloff, D. Van Derveer, J. Org. Chem. 1980, 45, 3545;
- 14dH. O. House, R. J. Outcalt, M. D. Cliffton, J. Org. Chem. 1982, 47, 2413;
- 14eH. O. House, R. J. Outcalt, J. L. Haack, D. Van Derveer, J. Org. Chem. 1983, 48, 1654;
- 14fK. A. Campbell, H. O. House, B. W. Surber, W. S. Trahanovsky, J. Org. Chem. 1987, 52, 2474;
- 14gG. A. Kraus, Y.-S. Hon, J. Org. Chem. 1986, 51, 116;
- 14hG. A. Kraus, P. Yi, Synth. Commun. 1988, 18, 473;
- 14iG. A. Kraus, Y.-S. Hon, J. Am. Chem. Soc. 1985, 107, 4341.
- 15
- 15aH. O. House, J. L. Haack, W. C. McDaniel, D. Van Derveer, J. Org. Chem. 1983, 48, 1643;
- 15bH. J. Bestmann, G. Schade, Tetrahedron Lett. 1982, 23, 3543.
- 16S. Chow, C. Kreß, N. Albæk, C. Jessen, C. M. Williams, Org. Lett. 2011, 13, 5286.
- 17E. W. Della, J. Tsanaktsidis, Aust. J. Chem. 1989, 42, 61.
- 18R. J. Giguere, T. L. Bray, S. M. Duncan, G. Majetich, Tetrahedron Lett. 1986, 27, 4945.
- 19For selected references on the Diels–Alder reaction with 18, see:
- 19aD. M. Hollinshead, S. C. Howell, S. V. Ley, M. Mahon, N. M. Ratcliffe, P. A. Worthington, J. Chem. Soc. Perkin Trans. 1 1983, 1579;
- 19bT. A. Engler, S. Naganathan, F. Takusagawa, D. Yohannes, Tetrahedron Lett. 1987, 28, 5267;
- 19cT. A. Engler, S. Naganathan, Tetrahedron Lett. 1986, 27, 1015;
- 19dT. A. Engler, U. Sampath, S. Naganathan, D. Vander Velde, F. Takusagawa, D. Yohannes, J. Org. Chem. 1989, 54, 5712;
- 19eT. A. Engler, U. Sampath, D. V. Velde, F. Takusagawa, Tetrahedron 1992, 48, 9399;
- 19fJ. Knol, A. Meetsma, B. L. Feringa, Tetrahedron: Asymmetry 1995, 6, 1069;
- 19gA. Gorgues, D. Stephan, A. Belyasmine, A. Khanous, A. Le Coq, Tetrahedron 1990, 46, 2817;
- 19hM. C. Carreño, J. L. G. Ruano, M. A. Toledo, Chem. Eur. J. 2000, 6, 288;
10.1002/(SICI)1521-3765(20000117)6:2<288::AID-CHEM288>3.0.CO;2-2 CAS PubMed Web of Science® Google Scholar
- 19iT. Mayelvaganan, S. B. Hadimani, S. V. Bhat, Tetrahedron 1997, 53, 2185;
- 19jR. P. Hsung, J. Org. Chem. 1997, 62, 7904;
- 19kR. M. Kamble, M. M. V. Ramana, Helv. Chim. Acta 2011, 94, 261;
- 19lE. Tzouma, I. Mavridis, V. P. Vidali, A. N. Pitsinos, Tetrahedron Lett. 2016, 57, 3643.
- 20For recent reviews on natural product synthesis through Diels–Alder reactions, see:
- 20aJ. Han, X. Alexander, X. Lei, Synthesis 2015, 47, 1519;
- 20bC. C. Nawrat, C. J. Moody, Angew. Chem. Int. Ed. 2014, 53, 2056; Angew. Chem. 2014, 126, 2086;
- 20cP. T. Parvatkar, H. K. Kadam, S. G. Tilve, Tetrahedron 2014, 70, 2857;
- 20dC. Y. Wan, J. Deng, H. Liu, M. Bian, A. Li, Sci. China Chem. 2014, 57, 926.
- 21For recent reviews on the [2+2] photocycloaddition, see:
- 21aS. Poplata, A. Trçster, Y.-Q. Zou, T. Bach, Chem. Rev. 2016, 116, 9748;
- 21bM. D. Kärkäs, J. A. Porco, C. R. J. Stephenson, Chem. Rev. 2016, 116, 9683;
- 21cT. Bach, J. P. Hehn, Angew. Chem. Int. Ed. 2011, 50, 1000; Angew. Chem. 2011, 123, 1032;
- 21dN. Hoffmann, Chem. Rev. 2008, 108, 1052;
- 21eJ. Iriondo-Alberdi, M. F. Greaney, Eur. J. Org. Chem. 2007, 4801.
- 22A. C. Pinto, R. Pinchin, S. K. Prado, Phytochemistry 1983, 22, 2017.
- 23
- 23aF. Nagashima, M. Kondoh, T. Uematsu, A. Nishiyama, S. Saito, M. Sato, Y. Asakawa, Chem. Pharm. Bull. 2002, 50, 808;
- 23bM. Node, T. Kajimoto, E. Fujita, K. Fuji, Bull. Inst. Chem. Res. Kyoto Univ. 1987, 65, 129.
- 24T. J. A. Graham, T. H. Poole, C. N. Reese, B. C. Goess, J. Org. Chem. 2011, 76, 4132.
- 25M. M. Midland, A. Tramontano, S. A. Zderic, J. Organomet. Chem. 1978, 156, 203.
- 26H. C. Brown, N. N. Joshi, J. Org. Chem. 1988, 53, 4059.
- 27
- 27aP. A. Grieco, S. Gilman, M. Nishizawa, J. Org. Chem. 1976, 41, 1485;
- 27bT. W. Lee, E. J. Corey, J. Am. Chem. Soc. 2001, 123, 1872.
- 28
- 28aR. A. Benkeser, A. Rappa, L. A. Wolsieffer, J. Org. Chem. 1986, 51, 3391;
- 28bZ. Zhang, Y. Li, D. Zhao, Y. He, J. Gong, Z. Yang, Chem. Eur. J. 2017, 23, 1258.
- 29CCDC 1941855 contains the supplementary crystallographic data for compound 27. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.