Asymmetric Synthesis of Heterocyclic γ-Amino-Acid and Diamine Derivatives by Three-Component Radical Cascade Reactions
Dr. Danqing Zheng
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität, Corrensstrasse 40, 48149 Münster, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Armido Studer
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität, Corrensstrasse 40, 48149 Münster, Germany
Search for more papers by this authorDr. Danqing Zheng
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität, Corrensstrasse 40, 48149 Münster, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Armido Studer
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität, Corrensstrasse 40, 48149 Münster, Germany
Search for more papers by this authorGraphical Abstract
Abstract
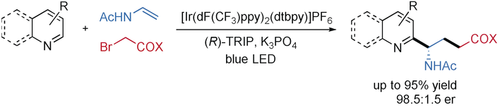
An enantioselective three-component radical reaction of quinolines or pyridines with enamides and α-bromo carbonyl compounds by dual photoredox and chiral Brønsted acid catalysis is presented. A range of valuable chiral γ-amino-acid derivatives are accessible in high chemo-, regio-, and enantioselectivity from simple, readily available starting materials under mild reaction conditions. Using the same strategy, the asymmetric synthesis of 1,2-diamine derivatives is also reported.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201908987-sup-0001-misc_information.pdf5.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. Trabocchi, F. Guarna, A. Guarna, Curr. Org. Chem. 2005, 9, 1127;
- 1bP. G. Vasudev, S. Chatterjee, N. Shamala, P. Balaram, Chem. Rev. 2011, 111, 657;
- 1cP. Conti, L. Tamborini, A. Pinto, A. Blondel, P. Minoprio, A. Mozzarelli, C. De Micheli, Chem. Rev. 2011, 111, 6919;
- 1dG. A. R. Johnston, Curr. Top. Med. Chem. 2002, 2, 903;
- 1eG. Costantino, A. Macchiarulo, A. E. Guadix, R. Pellicciari, J. Med. Chem. 2001, 44, 1827.
- 2J. Farrera-Sinfreu, E. Giralt, S. Castel, F. Albericio, M. Royo, J. Am. Chem. Soc. 2005, 127, 9495.
- 3
- 3aE. Roberts, Biochem. Pharmacol. 1974, 23, 2637;
- 3bI. A. Sytinsky, A. T. Soldatenkov, Prog. Neurobiol. 1978, 10, 89;
- 3cG. A. R. Johnston, Pharmacol. Ther. 1996, 69, 173.
- 4
- 4aP. Yuen, G. D. Kanter, C. P. Taylor, M. G. Vartanian, Bioorg. Med. Chem. Lett. 1994, 4, 823;
- 4bM. S. Hoekstra, D. M. Sobieray, M. A. Schwindt, T. A. Mulhern, T. M. Grote, B. K. Huckabee, V. S. Hendrickson, L. C. Franklin, E. J. Granger, G. L. Karrick, Org. Process Res. Dev. 1977, 1, 26;
- 4cG. J. Sills, Curr. Opin. Pharmacol. 2006, 6, 108.
- 5B. Lippert, B. W. Metcalf, M. J. Jung, P. Casara, Eur. J. Biochem. 1977, 74, 441.
- 6
- 6aB. A. Sachais, J. N. Logue, M. S. Carey, Arch. Neurol. 1977, 34, 422;
- 6bG. H. Fromm, C. F. Terrence, A. S. Chattha, J. D. Glass, Arch. Neurol. 1980, 37, 768.
- 7I. Lapin, CNS Drug Rev. 2001, 7, 471.
- 8J. Zhu, E. Mix, B. Winblad, CNS Drug Rev. 2001, 7, 387.
- 9For reviews, see:
- 9aM. Ordóñez, C. Cativiela, Tetrahedron: Asymmetry 2007, 18, 3;
- 9bK. Maruoka, T. Ooi, Chem. Rev. 2003, 103, 3013.
- 10For selected examples, see:
- 10aK. Akagawa, K. Kudo, Angew. Chem. Int. Ed. 2012, 51, 12786; Angew. Chem. 2012, 124, 12958;
- 10bT. Ooi, S. Fujioka, K. Maruoka, J. Am. Chem. Soc. 2004, 126, 11790;
- 10cJ. H. Sim, C. E. Song, Angew. Chem. Int. Ed. 2017, 56, 1835; Angew. Chem. 2017, 129, 1861;
- 10dR. Kastl, H. Wennemers, Angew. Chem. Int. Ed. 2013, 52, 7228; Angew. Chem. 2013, 125, 7369;
- 10eY. Chi, L. Guo, N. A. Kopf, S. H. Gellman, J. Am. Chem. Soc. 2008, 130, 5608;
- 10fA. Baschieri, L. Bernardi, A. Ricci, S. Suresh, M. F. A. Adamo, Angew. Chem. Int. Ed. 2009, 48, 9342; Angew. Chem. 2009, 121, 9506;
- 10gT. Okino, Y. Hoashi, T. Furukawa, X. Xu, Y. Takemoto, J. Am. Chem. Soc. 2005, 127, 119;
- 10hM. Wiesner, J. D. Revell, S. Tonazzi, H. Wennemers, J. Am. Chem. Soc. 2008, 130, 5610;
- 10iL. Guo, Y. Chi, A. M. Almeida, I. A. Guzei, B. K. Parker, S. H. Gellman, J. Am. Chem. Soc. 2009, 131, 16018.
- 11For selected examples, see:
- 11aL.-T. Shen, L.-H. Sun, S. Ye, J. Am. Chem. Soc. 2011, 133, 15894;
- 11bY.-Y. Zhou, L.-J. Wang, J. Li, X.-L. Sun, Y. Tang, J. Am. Chem. Soc. 2012, 134, 9066;
- 11cX.-Y. Chen, F. Xia, J.-T. Cheng, S. Ye, Angew. Chem. Int. Ed. 2013, 52, 10644; Angew. Chem. 2013, 125, 10838;
- 11dY.-Q. Fang, P. M. Tadross, E. N. Jacobsen, J. Am. Chem. Soc. 2014, 136, 17966;
- 11eC. Appayee, A. J. Fraboni, S. E. Brenner-Moyer, J. Org. Chem. 2012, 77, 8828;
- 11fJ. E. Gómez, W. Guo, S. Gaspa, A. W. Kleij, Angew. Chem. Int. Ed. 2017, 56, 15035; Angew. Chem. 2017, 129, 15231.
- 12One radical pathway used chiral auxiliaries for the asymmetric synthesis of γ-amino esters: G. K. Friestad, K. Banerjee, Org. Lett. 2009, 11, 1095.
- 13For selected reviews, see:
- 13aS. R. Chemler, P. H. Fuller, Chem. Soc. Rev. 2007, 36, 1153;
- 13bT. E. Müller, K. C. Hultzsch, M. Yus, F. Foubelo, M. Tada, Chem. Rev. 2008, 108, 3795;
- 13cR. I. McDonald, G. Liu, S. S. Stahl, Chem. Rev. 2011, 111, 2981;
- 13dJ. P. Wolfe, Angew. Chem. Int. Ed. 2012, 51, 10224; Angew. Chem. 2012, 124, 10370;
- 13eS. Tang, K. Liu, C. Liu, A. Lei, Chem. Soc. Rev. 2015, 44, 1070;
- 13fX. Zeng, Chem. Rev. 2013, 113, 6864;
- 13gR. W. Hoffmann, Chem. Soc. Rev. 2016, 45, 577;
- 13hJ. R. Coombs, J. P. Morken, Angew. Chem. Int. Ed. 2016, 55, 2636; Angew. Chem. 2016, 128, 2682.
- 14For a review in enantioselective radical processes, see:
- 14aM. P. Sibi, S. Manyem, J. Zimmerman, Chem. Rev. 2003, 103, 3263; Selected examples for intramolecular reactions:
- 14bR. Zhu, S. L. Buchwald, J. Am. Chem. Soc. 2015, 137, 8069;
- 14cY. Wang, L. Deng, J. Zhou, X. Wang, H. Mei, J. Han, Y. Pan, Adv. Synth. Catal. 2018, 360, 1060;
- 14dR. Zhu, S. L. Buchwald, Angew. Chem. Int. Ed. 2013, 52, 12655; Angew. Chem. 2013, 125, 12887;
- 14eJ.-S. Lin, X.-Y. Dong, T.-T. Li, N.-C. Jiang, B. Tan, X.-Y. Liu, J. Am. Chem. Soc. 2016, 138, 9357;
- 14fY.-F. Cheng, X.-Y. Dong, Q.-S. Gu, Z.-L. Yu, X.-Y. Liu, Angew. Chem. Int. Ed. 2017, 56, 8883; Angew. Chem. 2017, 129, 9009;
- 14gJ.-S. Lin, F.-L. Wang, X.-Y. Dong, W.-W. He, Y. Yuan, S. Chen, X.-Y. Liu, Nat. Commun. 2017, 8, 14841;
- 14hF.-L. Wang, X.-Y. Dong, J.-S. Lin, Y. Zeng, G.-Y. Jiao, Q.-S. Gu, X.-Q. Guo, C.-L. Ma, X.-Y. Liu, Chem 2017, 3, 979;
- 14iX.-T. Li, Q.-S. Gu, X.-Y. Dong, X. Meng, X.-Y. Liu, Angew. Chem. Int. Ed. 2018, 57, 7668; Angew. Chem. 2018, 130, 7794.
- 15
- 15aF. Wang, D. Wang, X. Wan, L. Wu, P. Chen, G. Liu, J. Am. Chem. Soc. 2016, 138, 15547;
- 15bL. Wu, F. Wang, X. Wan, D. Wang, P. Chen, G. Liu, J. Am. Chem. Soc. 2017, 139, 2904;
- 15cD. Wang, L. Wu, F. Wang, X. Wan, P. Chen, Z. Lin, G. Liu, J. Am. Chem. Soc. 2017, 139, 6811;
- 15dD. Wang, F. Wang, P. Chen, Z. Lin, G. Liu, Angew. Chem. Int. Ed. 2017, 56, 2054; Angew. Chem. 2017, 129, 2086;
- 15eF. Wang, D. Wang, Y. Zhou, L. Liang, R. Lu, P. Chen, Z. Lin, G. Liu, Angew. Chem. Int. Ed. 2018, 57, 7140; Angew. Chem. 2018, 130, 7258.
- 16J.-S. Lin, T.-T. Li, J.-R. Liu, G.-Y. Jiao, Q.-S. Gu, J.-T. Cheng, Y.-L. Guo, X. Hong, X.-Y. Liu, J. Am. Chem. Soc. 2019, 141, 1074.
- 17
- 17aR. S. J. Proctor, H. J. Davis, R. J. Phipps, Science 2018, 360, 419;
- 17bX. Liu, Y. Liu, G. Chai, B. Qiao, X. Zhao, Z. Jiang, Org. Lett. 2018, 20, 6298;
- 17cM.-C. Fu, R. Shang, B. Zhao, B. Wang, Y. Fu, Science 2019, 363, 1429; For racemic Minisci reactions:
- 17dM. A. J. Duncton, MedChemComm 2011, 2, 1135;
- 17eJ. Tauber, D. Imbri, T. Opatz, Molecules 2014, 19, 16190;
- 17fR. S. J. Proctor, R. J. Phipps, Angew. Chem. Int. Ed. 2019, 58, 13666; Angew. Chem. 2019, 131, 13802;
- 17gF. Minisci, R. Bernardi, F. Bertini, R. Galli, M. Perchinunno, Tetrahedron 1971, 27, 3575;
- 17hJ. Jin, D. W. C. MacMillan, Nature 2015, 525, 87;
- 17iJ. Jin, D. W. C. MacMillan, Angew. Chem. Int. Ed. 2015, 54, 1565; Angew. Chem. 2015, 127, 1585;
- 17jG.-X. Li, C. A. Morales-Rivera, Y. Wang, F. Gao, G. He, P. Liu, G. Chen, Chem. Sci. 2016, 7, 6407;
- 17kR. A. Garza-Sanchez, A. Tlahuext-Aca, G. Tavakoli, F. Glorius, ACS Catal. 2017, 7, 4057;
- 17lW.-M. Cheng, R. Shang, Y. Fu, ACS Catal. 2017, 7, 907;
- 17mW.-M. Cheng, R. Shang, M.-C. Fu, Y. Fu, Chem. Eur. J. 2017, 23, 2537;
- 17nZ. Liu, Z.-Q. Liu, Org. Lett. 2017, 19, 5649;
- 17oT. McCallum, L. Barriault, Chem. Sci. 2016, 7, 4754.
- 18D. Zheng, A. Studer, Org. Lett. 2019, 21, 325.
- 19CCDC 1940672 (4 e) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 20For selected reviews on the importance of chiral 1,2-diamines, see:
- 20aJ.-C. Kizirian, Chem. Rev. 2008, 108, 140;
- 20bD. Lucet, T. Le Gall, C. Mioskowski, Angew. Chem. Int. Ed. 1998, 37, 2580;
10.1002/(SICI)1521-3773(19981016)37:19<2580::AID-ANIE2580>3.0.CO;2-L CAS PubMed Web of Science® Google ScholarAngew. Chem. 1998, 110, 2724.10.1002/(SICI)1521-3757(19981002)110:19<2724::AID-ANGE2724>3.0.CO;2-4 Web of Science® Google Scholar
- 21H. G. Roth, N. A. Romero, D. A. Nicewicz, Synlett 2016, 27, 714.
- 22C. K. Prier, D. A. Rankic, D. W. C. MacMillan, Chem. Rev. 2013, 113, 5322.