Surface-Electron Coupling for Efficient Hydrogen Evolution
Weiwei Fu
The School of Chemistry and Chemical Engineering, State Key Laboratory of Power Transmission Equipment & System Security and New Technology, Chongqing University, 174 Shazheng Street, Shapingba District, Chongqing City, 400044 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorYanwei Wang
The School of Chemistry and Chemical Engineering, State Key Laboratory of Power Transmission Equipment & System Security and New Technology, Chongqing University, 174 Shazheng Street, Shapingba District, Chongqing City, 400044 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorJisong Hu
School of Science, Hubei University of Technology, Wuhan, 430068 P. R. China
Search for more papers by this authorHuijuan Zhang
The School of Chemistry and Chemical Engineering, State Key Laboratory of Power Transmission Equipment & System Security and New Technology, Chongqing University, 174 Shazheng Street, Shapingba District, Chongqing City, 400044 P. R. China
Search for more papers by this authorPing Luo
The School of Chemistry and Chemical Engineering, State Key Laboratory of Power Transmission Equipment & System Security and New Technology, Chongqing University, 174 Shazheng Street, Shapingba District, Chongqing City, 400044 P. R. China
Search for more papers by this authorFang Sun
The School of Chemistry and Chemical Engineering, State Key Laboratory of Power Transmission Equipment & System Security and New Technology, Chongqing University, 174 Shazheng Street, Shapingba District, Chongqing City, 400044 P. R. China
Search for more papers by this authorXinguo Ma
School of Science, Hubei University of Technology, Wuhan, 430068 P. R. China
Search for more papers by this authorZhengyong Huang
The School of Electrical Engineering, Chongqing University, 174 Shazheng Street, Shapingba District, Chongqing City, 400044 P. R. China
Search for more papers by this authorJian Li
The School of Electrical Engineering, Chongqing University, 174 Shazheng Street, Shapingba District, Chongqing City, 400044 P. R. China
Search for more papers by this authorCorresponding Author
Zaiping Guo
Institute for Superconducting and Electronic Materials, Australian Institute for Innovative Materials, University of Wollongong, Innovation Campus, North Wollongong, NSW, 2500 Australia
Search for more papers by this authorCorresponding Author
Yu Wang
The School of Chemistry and Chemical Engineering, State Key Laboratory of Power Transmission Equipment & System Security and New Technology, Chongqing University, 174 Shazheng Street, Shapingba District, Chongqing City, 400044 P. R. China
The School of Electrical Engineering, Chongqing University, 174 Shazheng Street, Shapingba District, Chongqing City, 400044 P. R. China
Search for more papers by this authorWeiwei Fu
The School of Chemistry and Chemical Engineering, State Key Laboratory of Power Transmission Equipment & System Security and New Technology, Chongqing University, 174 Shazheng Street, Shapingba District, Chongqing City, 400044 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorYanwei Wang
The School of Chemistry and Chemical Engineering, State Key Laboratory of Power Transmission Equipment & System Security and New Technology, Chongqing University, 174 Shazheng Street, Shapingba District, Chongqing City, 400044 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorJisong Hu
School of Science, Hubei University of Technology, Wuhan, 430068 P. R. China
Search for more papers by this authorHuijuan Zhang
The School of Chemistry and Chemical Engineering, State Key Laboratory of Power Transmission Equipment & System Security and New Technology, Chongqing University, 174 Shazheng Street, Shapingba District, Chongqing City, 400044 P. R. China
Search for more papers by this authorPing Luo
The School of Chemistry and Chemical Engineering, State Key Laboratory of Power Transmission Equipment & System Security and New Technology, Chongqing University, 174 Shazheng Street, Shapingba District, Chongqing City, 400044 P. R. China
Search for more papers by this authorFang Sun
The School of Chemistry and Chemical Engineering, State Key Laboratory of Power Transmission Equipment & System Security and New Technology, Chongqing University, 174 Shazheng Street, Shapingba District, Chongqing City, 400044 P. R. China
Search for more papers by this authorXinguo Ma
School of Science, Hubei University of Technology, Wuhan, 430068 P. R. China
Search for more papers by this authorZhengyong Huang
The School of Electrical Engineering, Chongqing University, 174 Shazheng Street, Shapingba District, Chongqing City, 400044 P. R. China
Search for more papers by this authorJian Li
The School of Electrical Engineering, Chongqing University, 174 Shazheng Street, Shapingba District, Chongqing City, 400044 P. R. China
Search for more papers by this authorCorresponding Author
Zaiping Guo
Institute for Superconducting and Electronic Materials, Australian Institute for Innovative Materials, University of Wollongong, Innovation Campus, North Wollongong, NSW, 2500 Australia
Search for more papers by this authorCorresponding Author
Yu Wang
The School of Chemistry and Chemical Engineering, State Key Laboratory of Power Transmission Equipment & System Security and New Technology, Chongqing University, 174 Shazheng Street, Shapingba District, Chongqing City, 400044 P. R. China
The School of Electrical Engineering, Chongqing University, 174 Shazheng Street, Shapingba District, Chongqing City, 400044 P. R. China
Search for more papers by this authorGraphical Abstract
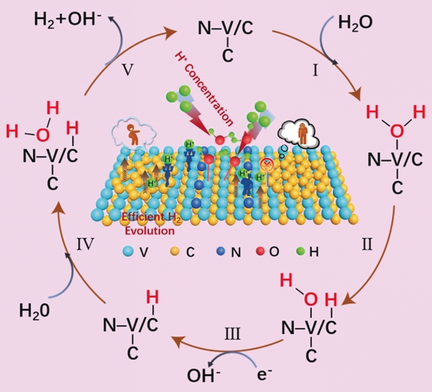
Small modifications have N effect: The controllable modification of graphene/V8C7 heterostructures by nitrogen is reported. Due to changes in the electronic structure of the different parts of the modified heterostructure, it displays an exceptional alkaline hydrogen-evolution capability, the most efficient alkaline hydrogen-evolution catalysis among transition-metal carbides reported thus far.
Abstract
Maximizing the activity of materials towards the alkaline hydrogen evolution reaction while maintaining their structural stability under realistic working conditions remains an area of active research. Herein, we report the first controllable surface modification of graphene(G)/V8C7 heterostructures by nitrogen. Because the introduced N atoms couple electronically with V atoms, the V sites can reduce the energy barrier for water adsorption and dissociation. Investigation of the multi-regional synergistic catalysis on N-modified G/V8C7 by experimental observations and density-functional-theory calculations reveals that the increase of electron density on the epitaxial graphene enable it to become favorable for H* adsorption and the subsequent reaction with another H2O molecule. This work extends the range of surface-engineering approaches to optimize the intrinsic properties of materials and could be generalized to the surface modification of other transition-metal carbides.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201908938-sup-0001-misc_information.pdf2.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1T. R. Hellstern, J. D. Benck, J. Kibsgaard, C. Hahn, T. F. Jaramillo, Adv. Energy Mater. 2016, 6, 1501758.
- 2J. K. Norskov, C. H. Christensen, Science 2006, 312, 1322–1323.
- 3
- 3aP. Rüetschi, P. Delahay, J. Chem. Phys. 1955, 23, 195–199;
- 3bB. E. Conway, J. O. M. Bockris, J. Chem. Phys. 1957, 26, 532–541;
- 3cJ. Mahmood, F. Li, S. M. Jung, M. S. Okyay, I. Ahmad, S. J. Kim, N. Park, H. Y. Jeong, J. B. Baek, Nat. Nanotechnol. 2017, 12, 441–446.
- 4
- 4aK. Zeng, D. Zhang, Prog. Energy Combust. Sci. 2010, 36, 307–326;
- 4bT. Abbasi, S. A. Abbasi, Renewable Sustainable Energy Rev. 2011, 15, 3034–3040.
- 5
- 5aJ. Durst, A. Siebel, C. Simon, F. Hasche, J. Herranz, H. A. Gasteiger, Energy Environ. Sci. 2014, 7, 2255–2260;
- 5bQ. Lu, G. S. Hutchings, W. Yu, Y. Zhou, R. V. Forest, R. Tao, J. Rosen, B. T. Yonemoto, Z. Cao, H. Zheng, J. Q. Xiao, F. Jiao, J. G. Chen, Nat. Commun. 2015, 6, 6567.
- 6J. Jiang, M. Gao, W. Sheng, Y. Yan, Angew. Chem. Int. Ed. 2016, 55, 15240–15245; Angew. Chem. 2016, 128, 15466–15471.
- 7
- 7aS. R. Chemler, M. T. Bovino, ACS Catal. 2013, 3, 1076–1091;
- 7bS. T. Hunt, T. Nimmanwudipong, Y. Roman-Leshkov, Angew. Chem. Int. Ed. 2014, 53, 5131–5136; Angew. Chem. 2014, 126, 5231–5236;
- 7cC. Wan, Y. N. Regmi, B. M. Leonard, Angew. Chem. Int. Ed. 2014, 53, 6407–6410; Angew. Chem. 2014, 126, 6525–6528.
- 8X. Peng, L. S. Hu, L. Wang, X. M. Zhang, J. J. Fu, K. F. Huo, L. Y. S. Lee, K. Y. Wong, P. K. Chu, Nano Energy 2016, 26, 603–609.
- 9Z. H. Pu, I. S. Amiinu, Z. K. Kou, W. Q. Li, S. C. Mu, Angew. Chem. Int. Ed. 2017, 56, 11559–11564; Angew. Chem. 2017, 129, 11717–11722.
- 10G. Q. He, Y. Song, K. Liu, A. Walter, S. Chen, S. W. Chen, ACS Catal. 2013, 3, 831–838.
- 11W. F. Chen, C. H. Wang, K. Sasaki, N. Marinkovic, W. Xu, J. T. Muckerman, Y. Zhu, R. R. Adzic, Energy Environ. Sci. 2013, 6, 943–951.
- 12
- 12aN. Han, K. R. Yang, Z. Lu, Y. Li, W. Xu, T. Gao, Z. Cai, Y. Zhang, V. S. Batista, W. Liu, X. Sun, Nat. Commun. 2018, 9, 924;
- 12bY. Zhao, K. Kamiya, K. Hashimoto, S. Nakanishi, Angew. Chem. Int. Ed. 2013, 52, 13638–13641; Angew. Chem. 2013, 125, 13883–13886.
- 13J. Deng, P. Ren, D. Deng, X. Bao, Angew. Chem. Int. Ed. 2015, 54, 2100–2104; Angew. Chem. 2015, 127, 2128–2132.
- 14
- 14aM. Yu, Z. Wang, C. Hou, Z. Wang, C. Liang, C. Zhao, Y. Tong, X. Lu, S. Yang, Adv. Mater. 2017, 29, 1602868;
- 14bT. Sun, J. Wang, X. Chi, Y. Lin, Z. Chen, X. Ling, C. Qiu, Y. Xu, L. Song, W. Chen, C. Su, ACS Catal. 2018, 8, 7585–7592;
- 14cR. Ye, P. del Angel-Vicente, Y. Liu, M. J. Arellano-Jimenez, Z. Peng, T. Wang, Y. Li, B. I. Yakobson, S. H. Wei, M. J. Yacaman, J. M. Tour, Adv. Mater. 2016, 28, 1427–1432;
- 14dY. Zheng, Y. Jiao, M. Jaroniec, S. Z. Qiao, Angew. Chem. Int. Ed. 2015, 54, 52–65; Angew. Chem. 2015, 127, 52–66.
- 15C. C. Yan, H. B. Li, Y. F. Ye, H. H. Wu, F. Cai, R. Si, J. P. Xiao, S. Miao, S. H. Xie, F. Yang, Y. S. Li, G. X. Wang, X. H. Bao, Energy Environ. Sci. 2018, 11, 1204–1210.
- 16W. Fu, Y. Wang, H. Zhang, M. He, L. Fang, X. Yang, Z. Huang, J. Li, X. Gu, Y. Wang, J. Catal. 2019, 369, 47–53.
- 17
- 17aT. Z. Huang, S. Mao, G. H. Zhou, Z. H. Wen, X. K. Huang, S. Q. Ci, J. H. Chen, Nanoscale 2014, 6, 9608–9613;
- 17bT. Z. Huang, J. M. Yu, J. T. Han, Z. L. Zhang, Y. Xing, C. L. Wen, X. Y. Wu, Y. H. Zhang, J. Power Sources 2015, 300, 483–490.
- 18X. H. Lu, M. H. Yu, T. Zhai, G. M. Wang, S. L. Xie, T. Y. Liu, C. L. Liang, Y. X. Tong, Y. Li, Nano Lett. 2013, 13, 2628–2633.
- 19
- 19aY. Li, X. Tan, S. Chen, X. Bo, H. Ren, S. C. Smith, C. Zhao, Angew. Chem. Int. Ed. 2019, 58, 461–466; Angew. Chem. 2019, 131, 471–476;
- 19bC. Wan, B. M. Leonard, Chem. Mater. Chem. Mater 2015, 27, 4281–4288.
- 20Y.-H. Fang, Z.-P. Liu, ACS Catal. 2014, 4, 4364–4376.
- 21
- 21aY. Q. Zhang, B. Ouyang, J. Xu, S. Chen, R. S. Rawat, H. J. Fan, Adv. Energy Mater. 2016, 6, 221;
- 21bY. Y. Chen, Y. Zhang, W. J. Jiang, X. Zhang, Z. Dai, L. J. Wan, J. S. Hu, ACS Nano 2016, 10, 8851–8860.
- 22B. Wang, Y. Liu, J. Ye, Phys. Scr. 2013, 88, 015301.
- 23D.-H. Lim, J. Wilcox, J. Phys. Chem. C 2012, 116, 3653–3660.
- 24F. Wang, J.-L. Dubois, W. Ueda, J. Catal. 2009, 268, 260–267.
- 25
- 25aV. M. Bermudez, J. T. Robinson, Langmuir 2011, 27, 11026–11036;
- 25bA. Agarwala, T. Subramani, A. Goldbourt, D. Danovich, R. Yerushalmi, Angew. Chem. Int. Ed. 2013, 52, 7415–7418; Angew. Chem. 2013, 125, 7563–7566.