Polyethylene Aerogels with Combined Physical and Chemical Crosslinking: Improved Mechanical Resilience and Shape-Memory Properties
Douriya Khedaioui
Univ Lyon. Université Claude Bernard Lyon 1, CPE Lyon, CNRS, UMR 5265, Chemistry, Catalysis, Polymers and Processes, 43 Bvd du 11 Novembre 1918, 69616 Villeurbanne, France
Search for more papers by this authorDr. Christophe Boisson
Univ Lyon. Université Claude Bernard Lyon 1, CPE Lyon, CNRS, UMR 5265, Chemistry, Catalysis, Polymers and Processes, 43 Bvd du 11 Novembre 1918, 69616 Villeurbanne, France
Search for more papers by this authorDr. Franck D'Agosto
Univ Lyon. Université Claude Bernard Lyon 1, CPE Lyon, CNRS, UMR 5265, Chemistry, Catalysis, Polymers and Processes, 43 Bvd du 11 Novembre 1918, 69616 Villeurbanne, France
Search for more papers by this authorCorresponding Author
Dr. Damien Montarnal
Univ Lyon. Université Claude Bernard Lyon 1, CPE Lyon, CNRS, UMR 5265, Chemistry, Catalysis, Polymers and Processes, 43 Bvd du 11 Novembre 1918, 69616 Villeurbanne, France
Search for more papers by this authorDouriya Khedaioui
Univ Lyon. Université Claude Bernard Lyon 1, CPE Lyon, CNRS, UMR 5265, Chemistry, Catalysis, Polymers and Processes, 43 Bvd du 11 Novembre 1918, 69616 Villeurbanne, France
Search for more papers by this authorDr. Christophe Boisson
Univ Lyon. Université Claude Bernard Lyon 1, CPE Lyon, CNRS, UMR 5265, Chemistry, Catalysis, Polymers and Processes, 43 Bvd du 11 Novembre 1918, 69616 Villeurbanne, France
Search for more papers by this authorDr. Franck D'Agosto
Univ Lyon. Université Claude Bernard Lyon 1, CPE Lyon, CNRS, UMR 5265, Chemistry, Catalysis, Polymers and Processes, 43 Bvd du 11 Novembre 1918, 69616 Villeurbanne, France
Search for more papers by this authorCorresponding Author
Dr. Damien Montarnal
Univ Lyon. Université Claude Bernard Lyon 1, CPE Lyon, CNRS, UMR 5265, Chemistry, Catalysis, Polymers and Processes, 43 Bvd du 11 Novembre 1918, 69616 Villeurbanne, France
Search for more papers by this authorGraphical Abstract
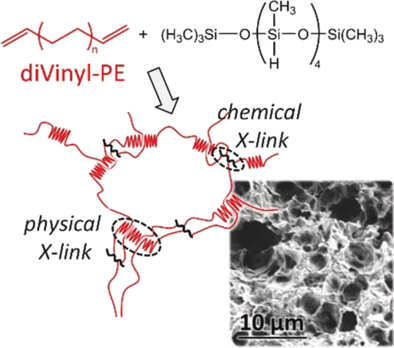
Taking shape: Polyethylene aerogels with low density and excellent mechanical resilience were obtained by combining and maximizing physical crosslinking, by crystallization, and chemical crosslinking, controlled by hydrosilylation reactions. These materials display remarkable shape-memory properties.
Abstract
While the introduction of polymers into aerogels strongly enhances their toughness, truly elastic monolithic aerogels which restore their dimensions upon extensive compression are still challenging to synthesize. In this context hydrophobic semi-crystalline polymers with low glass transition temperatures, and combined stiffness and flexibility, have only recently attracted attention. Shown here is that polyethylene aerogels with a low density, and combined chemical crosslinking and high crystallinity, display high moduli and excellent mechanical resilience. To maximize the crystallinity of these aerogels while maintaining a high crosslinking density, polyethylene networks with well-defined segments were synthesized by hydrosilylation crosslinking of telechelic, vinyl-functionalized oligomers obtained from catalyzed chain-growth polymerization. Recoverable deformations both above and below the melting temperature of polyethylene affords remarkable shape-memory properties.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201908257-sup-0001-misc_information.pdf1.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. C. Pierre, G. M. Pajonk, Chem. Rev. 2002, 102, 4243;
- 1bC. J. Brinker, C. J. Brinker, G. W. Scherer, Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing, Elsevier, Amsterdam, 1990.
- 2G. M. Pajonk, E. Elaloui, P. Achard, B. Chevalier, J.-L. Chevalier, M. Durant, J. Non-Cryst. Solids 1995, 186, 1.
- 3G. Zu, J. Shen, L. Zou, W. Wang, Y. Lian, Z. Zhang, A. Du, Chem. Mater. 2013, 25, 4757.
- 4M. A. Worsley, T. Y. Olson, J. R. Lee, T. M. Willey, M. H. Nielsen, S. K. Roberts, P. J. Pauzauskie, J. Biener, J. H. Satcher, Jr., T. F. Baumann, J. Phys. Chem. Lett. 2011, 2, 921.
- 5
- 5aA. Rigacci, J. Marechal, M. Repoux, M. Moreno, P. Achard, J. Non-Cryst. Solids 2004, 350, 372;
- 5bA. Bang, C. Buback, C. Sotiriou-Leventis, N. Leventis, Chem. Mater. 2014, 26, 6979.
- 6J. W. Steed, Chem. Commun. 2011, 47, 1379.
- 7T. Woignier, J. Phalippou, F. Despetis, S. Calas-Etienne, in Handbook of Sol-Gel Science and Technology: Processing, Characterization and Applications (Ed.: ), Springer International Publishing, Cham, 2018, pp. 985–1011.
10.1007/978-3-319-32101-1_27 Google Scholar
- 8H. Gesser, P. Goswami, Chem. Rev. 1989, 89, 765.
- 9S. A. Al-Muhtaseb, J. A. Ritter, Adv. Mater. 2003, 15, 101.
- 10
- 10aN. Diascorn, S. Calas, H. Sallée, P. Achard, A. Rigacci, J. Supercrit. Fluids 2015, 106, 76;
- 10bS. Donthula, C. Mandal, T. Leventis, J. Schisler, A. M. Saeed, C. Sotiriou-Leventis, N. Leventis, Chem. Mater. 2017, 29, 4461–4477.
- 11
- 11aZ. Wang, Z. Dai, J. Wu, N. Zhao, J. Xu, Adv. Mater. 2013, 25, 4494;
- 11bL. Jiang, K. Kato, K. Mayumi, H. Yokoyama, K. Ito, ACS Macro Lett. 2017, 6, 281;
- 11cL. Druel, R. Bardl, W. Vorwerg, T. Budtova, Biomacromolecules 2017, 18, 4232;
- 11dS. Salomo, T. X. Nguyen, D. K. Le, X. Zhang, N. Phan-Thien, H. M. Duong, Colloids Surf. A 2018, 556, 37;
- 11eS. Zhao, W. J. Malfait, A. Demilecamps, Y. Zhang, S. Brunner, L. Huber, P. Tingaut, A. Rigacci, T. Budtova, M. M. Koebel, Angew. Chem. Int. Ed. 2015, 54, 14282; Angew. Chem. 2015, 127, 14490.
- 12C. Rudaz, R. Courson, L. Bonnet, S. Calas-Etienne, H. Sallée, T. Budtova, Biomacromolecules 2014, 15, 2188.
- 13S. S. Silva, A. R. C. Duarte, J. F. Mano, R. L. Reis, Green Chem. 2013, 15, 3252.
- 14C. A. García-González, M. Alnaief, I. Smirnova, Carbohydr. Polym. 2011, 86, 1425.
- 15Z. Zhang, G. Sèbe, D. Rentsch, T. Zimmermann, P. Tingaut, Chem. Mater. 2014, 26, 2659.
- 16
- 16aC. Daniel, D. Alfano, V. Venditto, S. Cardea, E. Reverchon, D. Larobina, G. Mensitieri, G. Guerra, Adv. Mater. 2005, 17, 1515;
- 16bC. Daniel, S. Longo, R. Ricciardi, E. Reverchon, G. Guerra, Macromol. Rapid Commun. 2013, 34, 1194;
- 16cC. Daniel, J. G. Vitillo, G. Fasano, G. Guerra, ACS Appl. Mater. Interfaces 2011, 3, 969.
- 17
- 17aY. Zhang, D. Rodrigue, A. K. Abdellatif, J. Appl. Polym. Sci. 2003, 90, 2111–2119;
- 17bZ. Xing, G. Wu, S. Huang, S. Chen, H. Zeng, J. Supercrit. Fluids 2008, 47, 281–289.
- 18
- 18aW. Lu, Z. Yuan, Y. Zhao, H. Zhang, H. Zhang, X. Li, Chem. Soc. Rev. 2017, 46, 2199–2236;
- 18bP. Arora, Z. Zhang, Chem. Rev. 2004, 104, 4419–4469;
- 18cD. Ihm, J. Noh, J. Kim, J. Power Sources 2002, 109, 388–393.
- 19G. F. Müller, M. Stürzel, R. Mülhaupt, Adv. Funct. Mater. 2014, 24, 2860.
- 20C. Daniel, S. Longo, G. Guerra, Polyolefins J. 2015, 2, 49–55.
- 21Q. Xing, R. Li, X. Zhang, X. Dong, D. Wang, L. Zhang, Colloid Polym. Sci. 2015, 293, 3573.
- 22
- 22aJ. Mazzolini, E. Espinosa, F. D′Agosto, C. Boisson, Polym. Chem. 2010, 1, 793;
- 22bI. German, W. Kelhifi, S. Norsic, C. Boisson, F. D′Agosto, Angew. Chem. Int. Ed. 2013, 52, 3438; Angew. Chem. 2013, 125, 3522.
- 23S. Norsic, C. Thomas, F. D′Agosto, C. Boisson, Angew. Chem. Int. Ed. 2015, 54, 4631; Angew. Chem. 2015, 127, 4714.
- 24T. Sakai, T. Katashima, T. Matsushita, U. Chung, Polym. J. 2016, 48, 629.
- 25
- 25aY. Nakajima, S. Shimada, RSC Adv. 2015, 5, 20603;
- 25bA. Bouvet-Marchand, C. Chatard, A. Graillot, G. Boutevin, C. Loubat, D. Grosso, React. Chem. Eng. 2018, 3, 696.
- 26
- 26aI. E. Markó, S. Stérin, O. Buisine, G. Mignani, P. Branlard, B. Tinant, J.-P. Declercq, Science 2002, 298, 204;
- 26bI. E. Markó, S. Sterin, O. Buisine, G. Berthon, G. Michaud, B. Tinant, J.-P. Declercq, Adv. Synth. Catal. 2004, 346, 1429.
- 27W. Wu, J. Ke, M. Poliakoff, J. Chem. Eng. Data 2006, 51, 1398.
- 28I. Brückle, J. Thornton, K. Nichols, G. Strickler, J. Am. Inst. Conserv. 1999, 38, 162.
- 29W. Bald, J. Microsc. 1986, 143, 89.
- 30
- 30aL. J. Gibson, M. F. Ashby, Cellular Solids: Structure and Properties, Cambridge University Press, Cambridge, 1997;
10.1017/CBO9781139878326 Google Scholar
- 30bO. M. Istrate, B. Chen, Soft Matter 2011, 7, 1840.
- 31
- 31aT. Xie, Nature 2010, 464, 267;
- 31bC. Liu, H. Qin, P. T. Mather, J. Mater. Chem. 2007, 17, 1543–1558;
- 31cB. T. Michal, W. A. Brenn, B. N. Nguyen, L. S. McCorkle, M. A. B. Meador, S. J. Rowan, Chem. Mater. 2016, 28, 2341.
- 32
- 32aS. Malakooti, S. Rostami, H. G. Churu, H. Luo, J. Clark, F. Casarez, O. Rettenmaier, S. Daryadel, M. Minary-Jolandan, C. Sotiriou-Leventis, N. Leventis, H. Lu, RSC Adv. 2018, 8, 21214–21223;
- 32bS. Donthula, C. Mandal, J. Schisler, T. Leventis, M. A. B. Meador, C. Sotiriou-Leventis, N. Leventis, ACS Appl. Mater. Interfaces 2018, 10, 23321–23334.