Utilizing Carbonyl Coordination of Native Amides for Palladium-Catalyzed C(sp3)−H Olefination†
Hojoon Park
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorDr. Yang Li
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Jin-Quan Yu
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorHojoon Park
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorDr. Yang Li
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Jin-Quan Yu
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorA previous version of this manuscript has been deposited on a preprint server (https://doi.org/10.26434/chemrxiv.8135534.v1).
Graphical Abstract
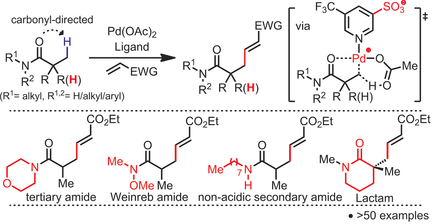
Go native: PdII-catalyzed C(sp3)−H olefination of native amide substrates was developed. To utilize the extremely weak carbonyl coordination ability of amides, an electron-deficient pyridinesulfonic acid ligand was designed. Structurally diverse amide substrates, including lactams, underwent olefination without the undesired cyclization. Olefination products were further diversified through synthetic transformations on the alkenyl moiety.
Abstract
PdII-catalyzed C(sp3)−H olefination of weakly coordinating native amides is reported. Three major drawbacks of previous C(sp3)−H olefination protocols, 1) in situ cyclization of products, 2) incompatibility with α-H-containing substrates, and 3) installation of exogenous directing groups, are addressed by harnessing the carbonyl coordination ability of amides to direct C(sp3)−H activation. The method enables direct C(sp3)−H functionalization of a wide range of native amide substrates, including secondary, tertiary, and cyclic amides, for the first time. The utility of this process is demonstrated by diverse transformations of the olefination products.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201906075-sup-0001-misc_information.pdf17.8 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. Mizoroki, K. Mori, A. Ozaki, Bull. Chem. Soc. Jpn. 1971, 44, 581;
- 1bR. F. Heck, J. P. Nolley, J. Org. Chem. 1972, 37, 2320.
- 2For reviews on the synthetic application of the Mizoroki–Heck reaction, see:
- 2aK. C. Nicolaou, P. G. Bulger, D. Sarlah, Angew. Chem. Int. Ed. 2005, 44, 4442; Angew. Chem. 2005, 117, 4516;
- 2bC. Torborg, M. Beller, Adv. Synth. Catal. 2009, 351, 3027.
- 3
- 3aL. Firmansjah, G. C. Fu, J. Am. Chem. Soc. 2007, 129, 11340;
- 3bK. S. Bloome, E. J. Alexanian, J. Am. Chem. Soc. 2010, 132, 12823.
- 4
- 4aF. Ozawa, T. Ito, A. Yamamoto, J. Am. Chem. Soc. 1980, 102, 6457;
- 4bA. Ariafard, Z. Lin, Organometallics 2006, 25, 4030; For a review on transition-metal catalysis with alkyl halides, see:
- 4cA. C. Frisch, M. Beller, Angew. Chem. Int. Ed. 2005, 44, 674; Angew. Chem. 2005, 117, 680.
- 5For examples using palladium catalysis, see:
- 5aK. S. Bloome, R. L. McMahen, E. J. Alexanian, J. Am. Chem. Soc. 2011, 133, 20146;
- 5bC. M. McMahon, E. J. Alexanian, Angew. Chem. Int. Ed. 2014, 53, 5974; Angew. Chem. 2014, 126, 6084;
- 5cD. Kurandina, M. Parasram, V. Gevorgyan, Angew. Chem. Int. Ed. 2017, 56, 14212; Angew. Chem. 2017, 129, 14400;
- 5dG.-Z. Wang, R. Shang, W.-M. Cheng, Y. Fu, J. Am. Chem. Soc. 2017, 139, 18307;
- 5eD. Kurandina, M. Rivas, M. Radzhabov, V. Gevorgyan, Org. Lett. 2018, 20, 357;
- 5fP. Chuentragool, D. Yadagiri, T. Morita, S. Sarkar, M. Parasram, Y. Wang, V. Gevorgyan, Angew. Chem. Int. Ed. 2019, 58, 1794; Angew. Chem. 2019, 131, 1808; For cobalt catalysis, see:
- 5gY. Ikeda, T. Nakamura, H. Yorimitsu, K. Oshima, J. Am. Chem. Soc. 2002, 124, 6514;
- 5hM. E. Weiss, L. M. Kreis, A. Lauber, E. M. Carreira, Angew. Chem. Int. Ed. 2011, 50, 11125; Angew. Chem. 2011, 123, 11321; For review, see:
- 5iD. Kurandina, P. Chuentragool, V. Gevorgyan, Synthesis 2019, 51, 985.
- 6
- 6aM. Wasa, K. M. Engle, J.-Q. Yu, J. Am. Chem. Soc. 2010, 132, 3680;
- 6bK. J. Stowers, K. C. Fortner, M. S. Sanford, J. Am. Chem. Soc. 2011, 133, 6541;
- 6cJ. He, S. Li, Y. Deng, H. Fu, B. N. Laforteza, J. E. Spangler, A. Homs, J.-Q. Yu, Science 2014, 343, 1216;
- 6dS. Li, G. Chen, C.-G. Feng, W. Gong, J.-Q. Yu, J. Am. Chem. Soc. 2014, 136, 5267;
- 6eH. Jiang, J. He, T. Liu, J.-Q. Yu, J. Am. Chem. Soc. 2016, 138, 2055;
- 6fW. Yang, S. Ye, Y. Schmidt, D. Stamos, J.-Q. Yu, Chem. Eur. J. 2016, 22, 7059;
- 6gN. Thrimurtulu, S. Khan, S. Maity, C. M. R. Volla, D. Maiti, Chem. Commun. 2017, 53, 12457;
- 6hZ. Zhuang, C.-B. Yu, G. Chen, Q.-F. Wu, Y. Hsiao, C. L. Joe, J. X. Qiao, M. A. Poss, J.-Q. Yu, J. Am. Chem. Soc. 2018, 140, 10363.
- 7J. He, M. Wasa, K. S. L. Chan, Q. Shao, J.-Q. Yu, Chem. Rev. 2017, 117, 8754.
- 8For reviews on C−H activation with weak coordination, see:
- 8aK. M. Engle, T.-S. Mei, M. Wasa, J.-Q. Yu, Acc. Chem. Res. 2012, 45, 788;
- 8bS. De Sarkar, W. Liu, S. I. Kozhushkov, L. Ackermann, Adv. Synth. Catal. 2014, 356, 1461.
- 9H. Park, N. Chekshin, P.-X. Shen, J.-Q. Yu, ACS Catal. 2018, 8, 9292.
- 10A. Greenberg, C. M. Breneman, J. F. Liebman, The Amide Linkage: Structural Significance in Chemistry, Biochemistry and Materials Science, Wiley, New York, 2000.
- 11
- 11aW. Gong, G. Zhang, T. Liu, R. Giri, J.-Q. Yu, J. Am. Chem. Soc. 2014, 136, 16940;
- 11bT. Liu, J. X. Qiao, M. A. Poss, J.-Q. Yu, Angew. Chem. Int. Ed. 2017, 56, 10924; Angew. Chem. 2017, 129, 11064;
- 11cA. F. M. Noisier, J. García, I. A. Ionuţ, F. Albericio, Angew. Chem. Int. Ed. 2017, 56, 314; Angew. Chem. 2017, 129, 320; For a recent review, see:
- 11dW. Wang, M. M. Lorion, J. Shah, A. R. Kapdi, L. Ackermann, Angew. Chem. Int. Ed. 2018, 57, 14700; Angew. Chem. 2018, 130, 14912.
- 12
- 12aM. Wasa, K. M. Engle, J.-Q. Yu, J. Am. Chem. Soc. 2009, 131, 9886;
- 12bY. Deng, W. Gong, J. He, J. Q. Yu, Angew. Chem. Int. Ed. 2014, 53, 6692; Angew. Chem. 2014, 126, 6810.
- 13T. Sato, K. Izawa, J. L. Aceña, H. Liu, V. A. Soloshonok, Eur. J. Org. Chem. 2016, 2757.
- 14
- 14aJ. J. Krutak, R. D. Burpitt, W. H. Moore, J. A. Hyatt, J. Org. Chem. 1979, 44, 3847;
- 14bQ. Chen, P. Mayer, H. Mayr, Angew. Chem. Int. Ed. 2016, 55, 12664; Angew. Chem. 2016, 128, 12854.
- 15J. Dong, L. Krasnova, M. G. Finn, K. B. Sharpless, Angew. Chem. Int. Ed. 2014, 53, 9430; Angew. Chem. 2014, 126, 9584.
- 16For examples of C(sp2)−H fluorosulfonyl vinylation, see:
- 16aC. Li, S.-M. Wang, H.-L. Qin, Org. Lett. 2018, 20, 4699;
- 16bX.-Y. Chen, Y. Wu, J. Zhou, P. Wang, J.-Q. Yu, Org. Lett. 2019, 21, 1426;
- 16cG. Ncube, M. P. Huestis, Organometallics 2019, 38, 76.
- 17For examples of C(sp2)−X fluorosulfonyl vinylation, see:
- 17aH. L. Qin, Q. Zheng, G. A. L. Bare, P. Wu, K. B. Sharpless, Angew. Chem. Int. Ed. 2016, 55, 14155; Angew. Chem. 2016, 128, 14361;
- 17bP. K. Chinthakindi, K. B. Govender, A. S. Kumar, H. G. Kruger, T. Govender, T. Naicker, P. I. Arvidsson, Org. Lett. 2017, 19, 480.
- 18A. J. Brouwer, N. Herrero Álvarez, A. Ciaffoni, H. van de Langemheen, R. M. J. Liskamp, Bioorg. Med. Chem. 2016, 24, 3429.