Site-Selective Occupancy of Eu2+ Toward Blue-Light-Excited Red Emission in a Rb3YSi2O7:Eu Phosphor
Jianwei Qiao
School of Materials Sciences and Engineering, University of Science and Technology Beijing, Beijing, 100083 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Lixin Ning
Anhui Key Laboratory of Optoelectric Materials Science and Technology, Key Laboratory of Functional Molecular Solids, Ministry of Education, Anhui Normal University, Wuhu, 241000 China
Search for more papers by this authorProf. Dr. Maxim S. Molokeev
Laboratory of Crystal Physics, Kirensky Institute of Physics, Federal Research Center KSC SB RAS, Krasnoyarsk, 660036 Russia
Siberian Federal University, Krasnoyarsk, 660041 Russia
Department of Physics, Far Eastern State Transport University, Khabarovsk, 680021 Russia
Search for more papers by this authorYu-Chun Chuang
National Synchrotron Radiation Research Center, Hsinchu, 300 Taiwan
Search for more papers by this authorProf. Dr. Qinyuan Zhang
State Key Laboratory of Luminescent Materials and Devices and Institute of Optical Communication Materials, South China University of Technology, Guangzhou, 510641 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Kenneth R. Poeppelmeier
Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL, 60208-3113 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhiguo Xia
School of Materials Sciences and Engineering, University of Science and Technology Beijing, Beijing, 100083 P. R. China
State Key Laboratory of Luminescent Materials and Devices and Institute of Optical Communication Materials, South China University of Technology, Guangzhou, 510641 China
Search for more papers by this authorJianwei Qiao
School of Materials Sciences and Engineering, University of Science and Technology Beijing, Beijing, 100083 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Lixin Ning
Anhui Key Laboratory of Optoelectric Materials Science and Technology, Key Laboratory of Functional Molecular Solids, Ministry of Education, Anhui Normal University, Wuhu, 241000 China
Search for more papers by this authorProf. Dr. Maxim S. Molokeev
Laboratory of Crystal Physics, Kirensky Institute of Physics, Federal Research Center KSC SB RAS, Krasnoyarsk, 660036 Russia
Siberian Federal University, Krasnoyarsk, 660041 Russia
Department of Physics, Far Eastern State Transport University, Khabarovsk, 680021 Russia
Search for more papers by this authorYu-Chun Chuang
National Synchrotron Radiation Research Center, Hsinchu, 300 Taiwan
Search for more papers by this authorProf. Dr. Qinyuan Zhang
State Key Laboratory of Luminescent Materials and Devices and Institute of Optical Communication Materials, South China University of Technology, Guangzhou, 510641 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Kenneth R. Poeppelmeier
Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL, 60208-3113 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhiguo Xia
School of Materials Sciences and Engineering, University of Science and Technology Beijing, Beijing, 100083 P. R. China
State Key Laboratory of Luminescent Materials and Devices and Institute of Optical Communication Materials, South China University of Technology, Guangzhou, 510641 China
Search for more papers by this authorGraphical Abstract
Rb3YSi2O7:Eu is used for phosphor-converted LEDs in solid-state lighting. It exhibits impressive properties, including an unprecedented blue-light-excited red emission in the oxide system. The results can initiate more exploration on how site-selective occupation design enables the discovery of blue-light-excited Eu2+-doped red-emitting oxide-based phosphors for white LEDs.
Abstract
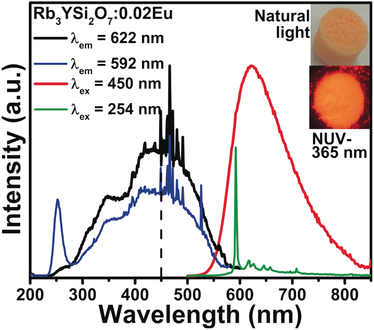
Establishing an effective design principle in solid-state materials for a blue-light-excited Eu2+-doped red-emitting oxide-based phosphors remains one of the significant challenges for white light-emitting diodes (WLEDs). Selective occupation of Eu2+ in inorganic polyhedra with small coordination numbers results in broad-band red emission as a result of enhanced crystal-field splitting of 5d levels. Rb3YSi2O7:Eu exhibits a broad emission band at λmax=622 nm under 450 nm excitation, and structural analysis and DFT calculations support the concept that Eu2+ ions preferably occupy RbO6 and YO6 polyhedra and show the characteristic red emission band of Eu2+. The excellent thermal quenching resistance, high color-rendering index Ra (93), and low CCT (4013 K) of the WLEDs clearly demonstrate that site engineering of rare-earth phosphors is an effective strategy to target tailored optical performance.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201905787-sup-0001-misc_information.pdf940.2 KB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aH. S. Jang, Y. H. Won, D. Y. Jeon, Appl. Phys. B 2009, 95, 715–720;
- 1bV. Bachmann, C. Ronda, A. Meijerink, Chem. Mater. 2009, 21, 2077–2084.
- 2
- 2aP. Pust, P. J. Schmidt, W. Schnick, Nat. Mater. 2015, 14, 454–458;
- 2bZ. Wang, J. Ha, Y. H. Kim, W. B. Im, J. McKittrick, S. P. Ong, Joule 2018, 2, 914–926.
- 3P. Pust, V. Weiler, C. Hecht, A. Tucks, A. S. Wochnik, A. K. Henss, D. Wiechert, C. Scheu, P. J. Schmidt, W. Schnick, Nat. Mater. 2014, 13, 891–896.
- 4C. Guo, D. Huang, Q. Su, Mater. Sci. Eng. B 2006, 130, 189–193.
- 5
- 5aK. Uheda, N. Hirosaki, Y. Yamamoto, A. Naito, T. Nakajima, H. Yamamoto, Electrochem. Solid-State Lett. 2006, 9, H 22–H25;
- 5bR.-J. Xie, N. Hirosaki, T. Suehiro, F.-F. Xu, M. Mitomo, Chem. Mater. 2006, 18, 5578–5583;
- 5cL. Wang, R.-J. Xie, Y. Li, X. Wang, C.-G. Ma, D. Luo, T. Takeda, Y.-T. Tsai, R.-S. Liu, N. Hirosaki, Light: Sci. Appl. 2016, 5, e 16155;
- 5dL. Chen, M. Fei, Z. Zhang, Y. Jiang, S. Chen, Y. Dong, Z. Sun, Z. Zhao, Y. Fu, J. He, C. Li, Z. Jiang, Chem. Mater. 2016, 28, 5505–5515;
- 5eJ. Häusler, L. Neudert, M. Mallmann, R. Niklaus, A. C. L. Kimmel, N. S. Alt, E. Schlücker, O. Oeckler, W. Schnick, Chem. Eur. J. 2017, 23, 2583–2590.
- 6
- 6aA. A. Setlur, E. V. Radkov, C. S. Henderson, J.-H. Her, A. M. Srivastava, N. Karkada, M. S. Kishore, N. P. Kumar, D. Aesram, A. Deshpande, B. Kolodin, L. S. Grigorov, U. Happek, Chem. Mater. 2010, 22, 4076–4082;
- 6bT. Takahashi, S. Adachi, J. Electrochem. Soc. 2008, 155, E 183–E188.
- 7H. Zhu, C. C. Lin, W. Luo, S. Shu, Z. Liu, Y. Liu, J. Kong, E. Ma, Y. Cao, R. S. Liu, X. Chen, Nat. Commun. 2014, 5, 4312.
- 8
- 8aY. Sato, H. Kato, M. Kobayashi, T. Masaki, D. H. Yoon, M. Kakihana, Angew. Chem. Int. Ed. 2014, 53, 7756–7759; Angew. Chem. 2014, 126, 7890–7893;
- 8bP. Zhang, L. Li, M. Xu, L. Liu, J. Alloys Compd. 2008, 456, 216–219;
- 8cT. Zhou, Z. Song, X. Song, L. Bian, Q. Liu, Chin. Phys. B 2010, 19, 127808.
- 9P. Dorenbos, J. Lumin. 2003, 104, 239–260.
- 10H. Daicho, Y. Shinomiya, K. Enomoto, A. Nakano, H. Sawa, S. Matsuishi, H. Hosono, Chem. Commun. 2018, 54, 884–887.
- 11F. J. DiSalvo, S. J. Clarke, Curr. Opin. Solid State Mater. Sci. 1996, 1, 241–249.
- 12
- 12aP. Dorenbos, ECS J. Solid State Sci. Technol. 2013, 2, R 3001–R3011;
- 12bS. Wang, Z. Song, Y. Kong, Z. Xia, Q. Liu, J. Lumin. 2018, 194, 461–466.
- 13TOPAS V.2: General profile and structure analysis software for powder diffraction data-User's Manual; Bruker AXS: Karlsruhe, Germany, 2008.
- 14A. M. Latshaw, G. Morrison, K. D. zur Loye, A. R. Myers, M. D. Smith, H.-C. zur Loye, CrystEngComm 2016, 18, 2294–2302.
- 15J.-F. Bérar, P. Lelann, J. Appl. Crystallogr. 1991, 24, 1–5.
- 16R. D. Shannon, Acta Crystallogr. Sect. A 1976, 32, 751–767.
- 17M. Zhao, Z. Xia, X. Huang, L. Ning, R. Gautier, M. S. Molokeev, Y. Zhou, Y.-C. Chuang, Q. Zhang, Q. Liu, Sci. Adv. 2019, 5, eaav 0363.
- 18B. Henderson, G. F. Imbusch, Optical spectroscopy of inorganic solids, Vol. 44, Oxford University Press, 2006.
10.1093/oso/9780199298624.001.0001 Google Scholar
- 19P. Dorenbos, J. Lumin. 2002, 99, 283–299.
- 20P. Dorenbos, J. Phys. Condens. Matter 2003, 15, 4797.
- 21A. Diaz, D. A. Keszler, Mater. Res. Bull. 1996, 31, 147–151.
- 22M. A. Lim, J. K. Park, C. H. Kim, H. D. Park, M. W. Han, J. Mater. Sci. Lett. 2003, 22, 1351–1353.
- 23
- 23aM. Zhao, H. Liao, L. Ning, Q. Zhang, Q. Liu, Z. Xia, Adv. Mater. 2018, 30, 1802489;
- 23bS. Ray, P. Tadge, S. J. Dhoble, G. B. Nair, A. Singh, A. K. Singh, M. Rai, T. M. Chen, V. Rajput, J. Alloys Compd. 2017, 713, 138–147;
- 23cM. Zhang, Y. Liang, S. Xu, Y. Zhu, X. Wu, S. Liu, CrystEngComm 2016, 18, 68–76;
- 23dJ. Wang, H. Lin, Q. Huang, G. Xiao, J. Xu, B. Wang, T. Hu, Y. Wang, J. Mater. Chem. C 2017, 5, 1789–1797;
- 23eH. Liao, M. Zhao, M. S. Molokeev, Q. Liu, Z. Xia, Angew. Chem. Int. Ed. 2018, 57, 11728–11731; Angew. Chem. 2018, 130, 11902–11905.
- 24
- 24aZ. Ci, M. Que, Y. Shi, G. Zhu, Y. Wang, Inorg. Chem. 2014, 53, 2195–2199;
- 24bL. He, Z. Song, X. Jia, Z. Xia, Q. Liu, Inorg. Chem. 2018, 57, 609–616.
- 25CCDC 1909273 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.