Perquinolines A–C: Unprecedented Bacterial Tetrahydroisoquinolines Involving an Intriguing Biosynthesis
Dr. Yuriy Rebets
Department of Pharmacy, Pharmaceutical Biotechnology, University of Saarland, Campus, Bld. C2 3, Saarbrucken, 66123 Germany
These authors contributed equally to this work.
Search for more papers by this authorDr. Suvd Nadmid
Department of Pharmacy, Pharmaceutical Biotechnology, University of Saarland, Campus, Bld. C2 3, Saarbrucken, 66123 Germany
These authors contributed equally to this work.
Search for more papers by this authorDr. Constanze Paulus
Department of Pharmacy, Pharmaceutical Biotechnology, University of Saarland, Campus, Bld. C2 3, Saarbrucken, 66123 Germany
Search for more papers by this authorCharlotte Dahlem
Department of Pharmacy, Pharmaceutical Biology, University of Saarland, Campus, Bld. C2 3, Saarbrucken, 66123 Germany
Search for more papers by this authorDr. Jennifer Herrmann
Department Microbial Natural Products, Helmholtz Institute for Pharmaceutical Research Saarland (HIPS), Campus, Bld. 8 1, Saarbrucken, 66123 Germany
Search for more papers by this authorDr. Harald Hübner
Department of Chemistry and Pharmacy, Friedrich-Alexander-Universität Erlangen-Nürnberg, Nikolaus-Fiebiger-Strasse 10, 91058 Erlangen, Germany
Search for more papers by this authorDr. Christian Rückert
Center for Biotechnology—CeBiTec, University of Bielefeld, Universitätsstraße 25, 33615 Bielefeld, Germany
Search for more papers by this authorProf. Dr. Alexandra K. Kiemer
Department of Pharmacy, Pharmaceutical Biology, University of Saarland, Campus, Bld. C2 3, Saarbrucken, 66123 Germany
Search for more papers by this authorProf. Dr. Peter Gmeiner
Department of Chemistry and Pharmacy, Friedrich-Alexander-Universität Erlangen-Nürnberg, Nikolaus-Fiebiger-Strasse 10, 91058 Erlangen, Germany
Search for more papers by this authorProf. Dr. Jörn Kalinowski
Center for Biotechnology—CeBiTec, University of Bielefeld, Universitätsstraße 25, 33615 Bielefeld, Germany
Search for more papers by this authorProf. Dr. Rolf Müller
Department Microbial Natural Products, Helmholtz Institute for Pharmaceutical Research Saarland (HIPS), Campus, Bld. 8 1, Saarbrucken, 66123 Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Andriy Luzhetskyy
Department of Pharmacy, Pharmaceutical Biotechnology, University of Saarland, Campus, Bld. C2 3, Saarbrucken, 66123 Germany
Department Microbial Natural Products, Helmholtz Institute for Pharmaceutical Research Saarland (HIPS), Campus, Bld. 8 1, Saarbrucken, 66123 Germany
Search for more papers by this authorDr. Yuriy Rebets
Department of Pharmacy, Pharmaceutical Biotechnology, University of Saarland, Campus, Bld. C2 3, Saarbrucken, 66123 Germany
These authors contributed equally to this work.
Search for more papers by this authorDr. Suvd Nadmid
Department of Pharmacy, Pharmaceutical Biotechnology, University of Saarland, Campus, Bld. C2 3, Saarbrucken, 66123 Germany
These authors contributed equally to this work.
Search for more papers by this authorDr. Constanze Paulus
Department of Pharmacy, Pharmaceutical Biotechnology, University of Saarland, Campus, Bld. C2 3, Saarbrucken, 66123 Germany
Search for more papers by this authorCharlotte Dahlem
Department of Pharmacy, Pharmaceutical Biology, University of Saarland, Campus, Bld. C2 3, Saarbrucken, 66123 Germany
Search for more papers by this authorDr. Jennifer Herrmann
Department Microbial Natural Products, Helmholtz Institute for Pharmaceutical Research Saarland (HIPS), Campus, Bld. 8 1, Saarbrucken, 66123 Germany
Search for more papers by this authorDr. Harald Hübner
Department of Chemistry and Pharmacy, Friedrich-Alexander-Universität Erlangen-Nürnberg, Nikolaus-Fiebiger-Strasse 10, 91058 Erlangen, Germany
Search for more papers by this authorDr. Christian Rückert
Center for Biotechnology—CeBiTec, University of Bielefeld, Universitätsstraße 25, 33615 Bielefeld, Germany
Search for more papers by this authorProf. Dr. Alexandra K. Kiemer
Department of Pharmacy, Pharmaceutical Biology, University of Saarland, Campus, Bld. C2 3, Saarbrucken, 66123 Germany
Search for more papers by this authorProf. Dr. Peter Gmeiner
Department of Chemistry and Pharmacy, Friedrich-Alexander-Universität Erlangen-Nürnberg, Nikolaus-Fiebiger-Strasse 10, 91058 Erlangen, Germany
Search for more papers by this authorProf. Dr. Jörn Kalinowski
Center for Biotechnology—CeBiTec, University of Bielefeld, Universitätsstraße 25, 33615 Bielefeld, Germany
Search for more papers by this authorProf. Dr. Rolf Müller
Department Microbial Natural Products, Helmholtz Institute for Pharmaceutical Research Saarland (HIPS), Campus, Bld. 8 1, Saarbrucken, 66123 Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Andriy Luzhetskyy
Department of Pharmacy, Pharmaceutical Biotechnology, University of Saarland, Campus, Bld. C2 3, Saarbrucken, 66123 Germany
Department Microbial Natural Products, Helmholtz Institute for Pharmaceutical Research Saarland (HIPS), Campus, Bld. 8 1, Saarbrucken, 66123 Germany
Search for more papers by this authorGraphical Abstract
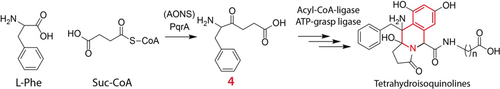
As easy as A,B,C: A new biosynthetic pathway for the tetrahydroisoquinolines perquinolines A–C starts with the condensation of succinyl-CoA and l-phenylalanine catalyzed by the AONS-like enzyme PqrA. The second condensation and cyclization events are mediated by the PqrG enzyme. An ATP-grasp RimK-type ligase PqrI completes the synthesis by transfer of a γ-aminobutyric acid or β-alanine moiety.
Abstract
Metabolic profiling of Streptomyces sp. IB2014/016-6 led to the identification of three new tetrahydroisoquinoline natural products, perquinolines A–C (1–3). Labelled precursor feeding studies and the cloning of the pqr biosynthetic gene cluster revealed that 1–3 are assembled by the action of several unusual enzymes. The biosynthesis starts with the condensation of succinyl-CoA and l-phenylalanine catalyzed by the amino-7-oxononanoate synthase-like enzyme PqrA, representing rare chemistry in natural product assembly. The second condensation and cyclization events are conducted by PqrG, an enzyme resembling an acyl-CoA ligase. Last, ATP-grasp RimK-type ligase PqrI completes the biosynthesis by transferring a γ-aminobutyric acid or β-alanine moiety. The discovered pathway represents a new route for assembling the tetrahydroisoquinoline cores of natural products.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201905538-sup-0001-misc_information.pdf4.7 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aP. L. Schiff, J. Nat. Prod. 1991, 54, 645–749;
- 1bJ. Stöckigt, A. P. Antonchick, F. R. Wu, H. Waldmann, Angew. Chem. Int. Ed. 2011, 50, 8538–8564; Angew. Chem. 2011, 123, 8692–8719;
- 1cC. Weber, T. Opatz, Alkaloids Chem. Biol. Perspect. 2019, 81, 1–114.
- 2D. Singla, A. Sharma, J. Kaur, B. Panwar, G. P. Raghava, BMC Pharmacol. 2010, 10, 4.
- 3J. D. Scott, R. M. Williams, Chem. Rev. 2002, 102, 1669–1730.
- 4V. H. Le, M. Inai, R. M. Williams, T. Kan, Nat. Prod. Rep. 2015, 32, 328–347.
- 5G. L. Tang, M. C. Tang, L. Q. Song, Y. Zhang, Curr. Top. Med. Chem. 2016, 16, 1717–1726.
- 6
- 6aJ. M. Finefield, D. H. Sherman, M. Kreitman, R. M. Williams, Angew. Chem. Int. Ed. 2012, 51, 4802–4836; Angew. Chem. 2012, 124, 4886–4920;
- 6bA. Bonamore, M. Barba, B. Botta, A. Boffi, A. Macone, Molecules 2010, 15, 2070–2078.
- 7K. Koketsu, K. Watanabe, H. Suda, H. Oguri, H. Oikawa, Nat. Chem. Biol. 2010, 6, 408–410.
- 8
- 8aA. Manglik, H. Lin, D. K. Aryal, J. D. McCorvy, D. Dengler, G. Corder, A. Levit, R. C. Kling, V. Bernat, H. Hubner, X. P. Huang, M. F. Sassano, P. M. Giguere, S. Lober, D. Duan, G. Scherrer, B. K. Kobilka, P. Gmeiner, B. L. Roth, B. K. Shoichet, Nature 2016, 537, 185;
- 8bD. M. Rosenbaum, C. Zhang, J. A. Lyons, R. Holl, D. Aragao, D. H. Arlow, S. G. F. Rasmussen, H. J. Choi, B. T. DeVree, R. K. Sunahara, P. S. Chae, S. H. Gellman, R. O. Dror, D. E. Shaw, W. I. Weis, M. Caffrey, P. Gmeiner, B. K. Kobilka, Nature 2011, 469, 236–240.
- 9M. Hashimoto, H. Komatsu, I. Kozone, H. Kawaide, H. Abe, M. Natsume, Biosci. Biotechnol. Biochem. 2005, 69, 315–320.
- 10T. Weber, K. Blin, S. Duddela, D. Krug, H. U. Kim, R. Bruccoleri, S. Y. Lee, M. A. Fischbach, R. Muller, W. Wohlleben, R. Breitling, E. Takano, M. H. Medema, Nucleic Acids Res. 2015, 43, W 237–W243.
- 11L. M. Iyer, S. Abhiman, A. M. Burroughs, L. Aravind, Mol. Biosyst. 2009, 5, 1636–1660.
- 12
- 12aV. Pfeifer, G. J. Nicholson, J. Ries, J. Recktenwald, A. B. Schefer, R. M. Shawky, J. Schroder, W. Wohlleben, S. Pelzer, J. Biol. Chem. 2001, 276, 38370–38377;
- 12bH. Chen, C. C. Tseng, B. K. Hubbard, C. T. Walsh, Proc. Natl. Acad. Sci. USA 2001, 98, 14901–14906.
- 13
- 13aP. M. Shoolingin-Jordan, S. Al-Daihan, D. Alexeev, R. L. Baxter, S. S. Bottomley, I. D. Kahari, I. Roy, M. Sarwar, L. Sawyer, S. F. Wang, Biochim. Biophys. Acta Proteins Proteomics 2003, 1647, 361–366;
- 13bS. Mann, O. Ploux, Biochim. Biophys. Acta Proteins Proteomics 2011, 1814, 1459–1466;
- 13cO. Bilyk, O. Sekurova, S. Zotchev, A. Luzhetskyy, Plos One 2016, 11, e 0158682.
- 14S. W. Chun, M. E. Hinze, M. A. Skiba, A. R. H. Narayan, J. Am. Chem. Soc. 2018, 140, 2430–2433.
- 15
- 15aB. Ostash, A. Saghatelian, S. Walker, Chem. Biol. 2007, 14, 257–267;
- 15bK. Petrickova, A. Chronakova, T. Zelenka, T. Chrudimsky, S. Pospisil, M. Petricek, V. Kristufek, Front. Microbiol. 2015, 6, 814.
- 16
- 16aI. Astner, J. O. Schulze, J. van den Heuvel, D. Jahn, W. D. Schubert, D. W. Heinz, EMBO J. 2005, 24, 3166–3177;
- 16bD. Alexeev, M. Alexeeva, R. L. Baxter, D. J. Campopiano, S. P. Webster, L. Sawyer, J. Mol. Biol. 1998, 284, 401–419.
- 17
- 17aA. Waterhouse, M. Bertoni, S. Bienert, G. Studer, G. Tauriello, R. Gumienny, F. T. Heer, T. A. P. de Beer, C. Rempfer, L. Bordoli, R. Lepore, T. Schwede, Nucleic Acids Res. 2018, 46, W 296–W303;
- 17bA. Schmidt, J. Sivaraman, Y. Li, R. Larocque, J. A. Barbosa, C. Smith, A. Matte, J. D. Schrag, M. Cygler, Biochemistry 2001, 40, 5151–5160.
- 18
- 18aN. M. Llewellyn, Y. Li, J. B. Spencer, Chem. Biol. 2007, 14, 379–386;
- 18bG. Zhao, Z. Jin, Y. Wang, N. M. Allewell, M. Tuchman, D. Shi, Proteins Struct. Funct. Bioinf. 2013, 81, 1847–1854;
- 18cK. Tabata, H. Ikeda, S. Hashimoto, J. Bacteriol. 2005, 187, 5195–5202;
- 18dJ. A. Baccile, J. E. Spraker, H. H. Le, E. Brandenburger, C. Gomez, J. W. Bok, J. Macheleidt, A. A. Brakhage, D. Hoffmeister, N. P. Keller, F. C. Schroeder, Nat. Chem. Biol. 2016, 12, 419-424.
- 19A. Pospiech, J. Bietenhader, T. Schupp, Microbiology 1996, 142, 741–746.