Molecular and Structural Characterization of a Promiscuous C-Glycosyltransferase from Trollius chinensis
Jun-Bin He
State Key Laboratory of Natural and Biomimetic Drugs & Key Laboratory of Molecular Cardiovascular Sciences of Ministry of Education, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing, 100191 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Peng Zhao
Department of Biochemistry and Biophysics &, Department of Integration of Chinese and Western Medicine, School of Basic Medical Sciences, Peking University, 38 Xueyuan Road, Beijing, 100191 China
These authors contributed equally to this work.
Search for more papers by this authorZhi-Min Hu
State Key Laboratory of Natural and Biomimetic Drugs & Key Laboratory of Molecular Cardiovascular Sciences of Ministry of Education, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing, 100191 China
Search for more papers by this authorShuang Liu
State Key Laboratory of Natural and Biomimetic Drugs & Key Laboratory of Molecular Cardiovascular Sciences of Ministry of Education, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing, 100191 China
Search for more papers by this authorYi Kuang
State Key Laboratory of Natural and Biomimetic Drugs & Key Laboratory of Molecular Cardiovascular Sciences of Ministry of Education, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing, 100191 China
Search for more papers by this authorMeng Zhang
State Key Laboratory of Natural and Biomimetic Drugs & Key Laboratory of Molecular Cardiovascular Sciences of Ministry of Education, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing, 100191 China
Search for more papers by this authorBin Li
State Key Laboratory of Natural and Biomimetic Drugs & Key Laboratory of Molecular Cardiovascular Sciences of Ministry of Education, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing, 100191 China
Search for more papers by this authorCorresponding Author
Prof. Cai-Hong Yun
Department of Biochemistry and Biophysics &, Department of Integration of Chinese and Western Medicine, School of Basic Medical Sciences, Peking University, 38 Xueyuan Road, Beijing, 100191 China
Search for more papers by this authorCorresponding Author
Dr. Xue Qiao
State Key Laboratory of Natural and Biomimetic Drugs & Key Laboratory of Molecular Cardiovascular Sciences of Ministry of Education, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing, 100191 China
Search for more papers by this authorCorresponding Author
Prof. Min Ye
State Key Laboratory of Natural and Biomimetic Drugs & Key Laboratory of Molecular Cardiovascular Sciences of Ministry of Education, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing, 100191 China
Search for more papers by this authorJun-Bin He
State Key Laboratory of Natural and Biomimetic Drugs & Key Laboratory of Molecular Cardiovascular Sciences of Ministry of Education, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing, 100191 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Peng Zhao
Department of Biochemistry and Biophysics &, Department of Integration of Chinese and Western Medicine, School of Basic Medical Sciences, Peking University, 38 Xueyuan Road, Beijing, 100191 China
These authors contributed equally to this work.
Search for more papers by this authorZhi-Min Hu
State Key Laboratory of Natural and Biomimetic Drugs & Key Laboratory of Molecular Cardiovascular Sciences of Ministry of Education, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing, 100191 China
Search for more papers by this authorShuang Liu
State Key Laboratory of Natural and Biomimetic Drugs & Key Laboratory of Molecular Cardiovascular Sciences of Ministry of Education, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing, 100191 China
Search for more papers by this authorYi Kuang
State Key Laboratory of Natural and Biomimetic Drugs & Key Laboratory of Molecular Cardiovascular Sciences of Ministry of Education, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing, 100191 China
Search for more papers by this authorMeng Zhang
State Key Laboratory of Natural and Biomimetic Drugs & Key Laboratory of Molecular Cardiovascular Sciences of Ministry of Education, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing, 100191 China
Search for more papers by this authorBin Li
State Key Laboratory of Natural and Biomimetic Drugs & Key Laboratory of Molecular Cardiovascular Sciences of Ministry of Education, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing, 100191 China
Search for more papers by this authorCorresponding Author
Prof. Cai-Hong Yun
Department of Biochemistry and Biophysics &, Department of Integration of Chinese and Western Medicine, School of Basic Medical Sciences, Peking University, 38 Xueyuan Road, Beijing, 100191 China
Search for more papers by this authorCorresponding Author
Dr. Xue Qiao
State Key Laboratory of Natural and Biomimetic Drugs & Key Laboratory of Molecular Cardiovascular Sciences of Ministry of Education, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing, 100191 China
Search for more papers by this authorCorresponding Author
Prof. Min Ye
State Key Laboratory of Natural and Biomimetic Drugs & Key Laboratory of Molecular Cardiovascular Sciences of Ministry of Education, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing, 100191 China
Search for more papers by this authorGraphical Abstract
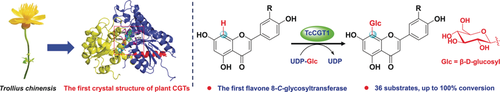
A promiscuous C-glycosyltransferase, TcCGT1, is highlighted. TcCGT1 represents the first flavone 8-C-glycosyltransferase that exhibits robust substrate promiscuity towards different types of flavonoids. The crystal structure of TcCGT1, the first crystal structure of a plant CGT, is shown. This work provides a basis for protein engineering to design efficient glycosylation biocatalysts for drug discovery.
Abstract
Herein, the catalytic promiscuity of TcCGT1, a new C-glycosyltransferase (CGT) from the medicinal plant Trollius chinensis is explored. TcCGT1 could efficiently and regio-specifically catalyze the 8-C-glycosylation of 36 flavones and other flavonoids and could also catalyze the O-glycosylation of diverse phenolics. The crystal structure of TcCGT1 in complex with uridine diphosphate was determined at 1.85 Å resolution. Molecular docking revealed a new model for the catalytic mechanism of TcCGT1, which is initiated by the spontaneous deprotonation of the substrate. The spacious binding pocket explains the substrate promiscuity, and the binding pose of the substrate determines C- or O-glycosylation activity. Site-directed mutagenesis at two residues (I94E and G284K) switched C- to O-glycosylation. TcCGT1 is the first plant CGT with a crystal structure and the first flavone 8-C-glycosyltransferase described. This provides a basis for designing efficient glycosylation biocatalysts.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201905505-sup-0001-misc_information.pdf14.6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aC. J. Thibodeaux, C. E. Melancon, H. W. Liu, Nature 2007, 446, 1008–1016;
- 1bC. J. Thibodeaux, C. E. Melancon III, H. W. Liu, Angew. Chem. Int. Ed. 2008, 47, 9814–9859; Angew. Chem. 2008, 120, 9960–10007;
- 1cT. Bililign, B. R. Griffith, J. S. Thorson, Nat. Prod. Rep. 2005, 22, 742–760.
- 2P. G. Hultin, Curr. Top. Med. Chem. 2005, 5, 1299–1331.
- 3
- 3aJ. Xiao, E. Capanoglu, A. R. Jassbi, A. Miron, Crit. Rev. Food Sci. Nutr. 2016, 56, S 29–S45;
- 3bO. Talhi, A. M. S. Silva, Curr. Org. Chem. 2012, 16, 859–896.
- 4
- 4aK. Y. Lam, A. P. K. Ling, R. Y. Koh, Y. P. Wong, Y. H. Say, Adv. Pharmacol. Sci. 2016, 4104595;
- 4bM. He, J. W. Min, W. L. Kong, X. H. He, J. X. Li, B. W. Peng, Fitoterapia 2016, 115, 74–85.
- 5S. Sato, T. Akiya, H. Nishizawa, T. Suzuki, Carbohydr. Res. 2006, 341, 964–970.
- 6
- 6aH. Satoh, S. Manabe, Chem. Soc. Rev. 2013, 42, 4297–4309;
- 6bY. Yang, B. Yu, Chem. Rev. 2017, 117, 12281–12356.
- 7
- 7aE. K. Lim, Chem. Eur. J. 2005, 11, 5486–5494;
- 7bC. Dürr, D. Hoffmeister, S. E. Wohlert, K. Ichinose, M. Weber, U. von Mulert, J. S. Thorson, A. Bechthold, Angew. Chem. Int. Ed. 2004, 43, 2962–2965; Angew. Chem. 2004, 116, 3022–3025;
- 7cD. W. Chen, R. D. Chen, R. S. Wang, J. H. Li, K. B. Xie, C. C. Bian, L. L. Sun, X. L. Zhang, J. M. Liu, L. Yang, F. Ye, X. M. Yu, J. G. Dai, Angew. Chem. Int. Ed. 2015, 54, 12678–12682; Angew. Chem. 2015, 127, 12869–12873.
- 8
- 8aM. Brazier-Hicks, K. M. Evans, M. C. Gershater, H. Puschmann, P. G. Steel, R. Edwards, J. Biol. Chem. 2009, 284, 17926–17934;
- 8bM. L. Hamilton, S. P. Kuate, M. Brazier-Hicks, J. C. Caulfield, R. Rose, R. Edwards, B. Torto, J. A. Pickett, A. M. Hooper, Phytochemistry 2012, 84, 169–176;
- 8cM. L. Falcone Ferreyra, E. Rodriguez, M. I. Casas, G. Labadie, E. Grotewold, P. Casati, J. Biol. Chem. 2013, 288, 31678–31688;
- 8dY. Nagatomo, S. Usui, T. Ito, A. Kato, M. Shimosaka, G. Taguchi, Plant J. 2014, 80, 437–448;
- 8eN. Sasaki, Y. Nishizaki, E. Yamada, F. Tatsuzawa, T. Nakatsuka, H. Takahashi, M. Nishihara, FEBS Lett. 2015, 589, 182–187;
- 8fY. Hirade, N. Kotoku, K. Terasaka, Y. Saijo-Hamano, A. Fukumoto, H. Mizukami, FEBS Lett. 2015, 589, 1778–1786;
- 8gB. Hao, J. C. Caulfield, M. L. Hamilton, J. A. Pickett, C. A. O. Midega, Z. R. Khan, J. Wang, A. M. Hooper, Phytochemistry 2016, 125, 73–87;
- 8hX. Wang, C. F. Li, C. Zhou, J. Li, Y. S. Zhang, Plant J. 2017, 90, 535–546;
- 8iT. Ito, S. Fujimoto, F. Suito, M. Shimosaka, G. Taguchi, Plant J. 2017, 91, 187–198.
- 9D. W. Chen, L. L. Sun, R. D. Chen, K. B. Xie, L. Yang, J. G. Dai, Chem. Eur. J. 2016, 22, 5873–5877.
- 10
- 10aH. Shao, X. Z. He, L. Achnine, J. W. Blount, R. A. Dixon, X. Q. Wang, Plant Cell 2005, 17, 3141–3154;
- 10bW. Offen, C. Martinez-Fleites, M. Yang, E. Kiat-Lim, B. G. Davis, C. A. Tarling, C. M. Ford, D. J. Bowles, G. J. Davies, EMBO J. 2006, 25, 1396–1405;
- 10cL. Li, L. V. Modolo, L. L. Escamilia-Trevino, L. Achnine, R. A. Dixon, X. Wang, J. Mol. Biol. 2007, 370, 951–963;
- 10dM. Brazier-Hicks, W. A. Offen, M. C. Gershater, T. J. Revett, E.-K. Lim, D. J. Bowles, G. J. Davies, R. Edwards, Proc. Natl. Acad. Sci. USA 2007, 104, 20238–20243;
- 10eT. Hiromoto, E. Honjo, N. Noda, T. Tamada, K. Kazuma, M. Suzuki, M. Blaber, R. Kuroki, Protein Sci. 2015, 24, 395–407;
- 10fL. V. Modolo, L. Li, H. Pan, J. W. Blount, R. A. Dixon, X. Wang, J. Mol. Biol. 2009, 392, 1292–1302;
- 10gM. Mittler, A. Bechthold, G. E. Schulz, J. Mol. Biol. 2007, 372, 67–76;
- 10hF. B. Wang, M. Q. Zhou, S. Singh, R. M. Yennamalli, C. A. Bingman, J. S. Thorson, G. N. Phillips, Proteins Struct. Funct. Bioinf. 2013, 81, 1277–1282;
- 10iD. Foshag, C. Campbell, P. D. Pawelek, Biochim. Biophys. Acta Proteins Proteomics 2014, 1844, 1619–1630.
- 11D. W. Chen, S. Fan, R. D. Chen, K. B. Xie, S. Yin, L. L. Sun, J. M. Liu, L. Yang, J. Q. Kong, Z. Y. Yang, J. G. Dai, ACS Catal. 2018, 8, 4917–4927.
- 12A. Gutmann, B. Nidetzky, Angew. Chem. Int. Ed. 2012, 51, 12879–12883; Angew. Chem. 2012, 124, 13051–13056.
- 13
- 13aM. Yuan, R. F. Wang, X. W. Wu, Y. N. An, X. W. Yang, Chin. J. Nat. Med. 2013, 11, 449–455;
- 13bE. Witkowska-Banaszczak, Phytother. Res. 2015, 29, 475–500.
- 14
- 14aL. Z. Wu, X. P. Zhang, X. D. Xu, Q. X. Zheng, J. S. Yang, W. L. Ding, J. Pharm. Biomed. Anal. 2013, 75, 55–63;
- 14bZ. L. Song, Y. Hashi, H. Y. Sun, Y. Liang, Y. X. Lan, H. Wang, S. Z. Chen, Fitoterapia 2013, 91, 272–279.
- 15
- 15aJ. X. Wei, D. Y. Li, Z. L. Li, Phytochem. Lett. 2018, 25, 156–162;
- 15bR. Yan, Y. Cui, B. Deng, J. Bi, G. Zhang, J. Nat. Med. 2019, 73, 297–302.
- 16X. Qiao, W. N. He, C. Xiang, J. Han, L. J. Wu, D. A. Guo, M. Ye, Phytochem. Anal. 2011, 22, 475–483.
- 17
- 17aK. Xie, R. Chen, J. Li, R. Wang, D. Chen, X. Dou, J. Dai, Org. Lett. 2014, 16, 4874–4877;
- 17bC. S. Zhang, B. R. Griffith, Q. Fu, C. Albermann, X. Fu, I. K. Lee, L. J. Li, J. S. Thorson, Science 2006, 313, 1291–1294.
- 18N. Krafczyk, M. A. Glomb, J. Agric. Food Chem. 2008, 56, 3368–3376.
- 19J. Napetschnig, H. Wu, Annu. Rev. Biophys. 2013, 42, 443–468.
- 20M. E. Turini, R. N. DuBois, Annu. Rev. Med. 2002, 53, 35–57.
- 21A. M. Mulichak, W. Lu, H. C. Losey, C. T. Walsh, R. M. Garavito, Biochemistry 2004, 43, 5170–5180.
- 22C. Breton, S. Fournel-Gigleux, M. M. Palcic, Curr. Opin. Struct. Biol. 2012, 22, 540–549.
- 23X. Robert, P. Gouet, Nucleic Acids Res. 2014, 42, W 320–W324.