Chemo- and Enantioselective Hydrogenation of α-Formyl Enamides: An Efficient Access to Chiral α-Amido Aldehydes
Jian Zhang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Pharmacy, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorJia Jia
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorXincheng Zeng
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Pharmacy, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorYuanhao Wang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Pharmacy, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorDr. Zhenfeng Zhang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Pharmacy, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorProf. Dr. Ilya D. Gridnev
Department of Chemistry, Graduate School of Science, Tohoku University, Aramaki 3–6, Aoba-ku, Sendai, 980-8578 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Wanbin Zhang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Pharmacy, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorJian Zhang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Pharmacy, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorJia Jia
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorXincheng Zeng
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Pharmacy, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorYuanhao Wang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Pharmacy, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorDr. Zhenfeng Zhang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Pharmacy, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorProf. Dr. Ilya D. Gridnev
Department of Chemistry, Graduate School of Science, Tohoku University, Aramaki 3–6, Aoba-ku, Sendai, 980-8578 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Wanbin Zhang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Pharmacy, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorGraphical Abstract
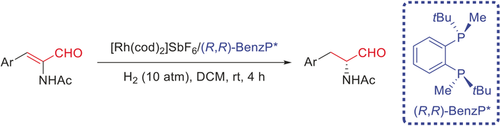
A new arrow in the quiver: A highly chemo- and enantioselective hydrogenation of α-formyl enamides has been developed for the synthesis of chiral α-amido aldehydes in high yields (98–99 %), with excellent chemo- and enantioselectivities (up to >99.9 % ee), and with high substrate/catalyst ratios (up to 20000 S/C). Computations give a clear description of the R/S pathways of the catalytic cycle.
Abstract
In order to effectively synthesize chiral α-amino aldehydes, which have a wide range of potential applications in organic synthesis and medicinal chemistry, a highly chemo- and enantioselective hydrogenation of α-formyl enamides has been developed, catalyzed by a rhodium complex of a P-stereogenic bisphosphine ligand. Under different hydrogen pressures, the chiral α-amido aldehydes and β-amido alcohols were obtained in high yields (97–99 %) and with excellent chemo- and enantioselectivities (up to >99.9 % ee). The hydrogenation can be carried out on a gram scale and with a high substrate/catalyst ratio (up to 20 000 S/C), and the hydrogenated products were further converted into several important chiral products. Computations of the catalytic cycle gave a clear description for the R/S pathways, provided a reasonable explanation for the enantioselectivity, and revealed several other specific features.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201905263-sup-0001-misc_information.pdf10.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Selected examples:
- 1aT. Lee, J. B. Jones, J. Am. Chem. Soc. 1996, 118, 502;
- 1bB. M. Trost, M. L. Crawley, C. B. Lee, J. Am. Chem. Soc. 2000, 122, 6120;
- 1cB. M. Trost, M. L. Crawley, J. Am. Chem. Soc. 2002, 124, 9328;
- 1dM. J. MacDonald, C. R. Hesp, D. J. Schipper, M. Pesant, A. M. Beauchemin, Chem. Eur. J. 2013, 19, 2597;
- 1eC. Blikstad, K. M. Dahlström, T. A. Salminen, M. Widersten, ACS Catal. 2013, 3, 3016;
- 1fJ. F. Hooper, S. Seo, F. R. Truscott, J. D. Neuhaus, M. C. Willis, J. Am. Chem. Soc. 2016, 138, 1630;
- 1gJ. Chen, X. Gong, J. Li, Y. Li, J. Ma, C. Hou, G. Zhao, W. Yuan, B. Zhao, Science 2018, 360, 1438;
- 1hW. Wen, L. Chen, M.-J. Luo, Y. Zhang, Y.-C. Chen, Q. Ouyang, Q.-X. Guo, J. Am. Chem. Soc. 2018, 140, 9774;
- 1iQ. Wang, Q. Gu, S.-L. You, Angew. Chem. Int. Ed. 2019, 58, 6818; Angew. Chem. 2019, 131, 6890.
- 2Review:
- 2aA. C. Brezny, C. R. Landis, Acc. Chem. Res. 2018, 51, 2344; Recent examples:
- 2bY. Deng, H. Wang, Y. Sun, X. Wang, ACS Catal. 2015, 5, 6828;
- 2cC. You, B. Wei, X. Li, Y. Yang, Y. Liu, H. Lv, X. Zhang, Angew. Chem. Int. Ed. 2016, 55, 6511; Angew. Chem. 2016, 128, 6621;
- 2dC. Schmitz, K. Holthusen, W. Leitner, G. Franciò, ACS Catal. 2016, 6, 1584;
- 2eC. You, S. Li, X. Li, J. Lan, Y. Yang, L. W. Chung, H. Lv, X. Zhang, J. Am. Chem. Soc. 2018, 140, 4977;
- 2fC. You, X. Li, Y. Yang, Y.-S. Yang, X. Tan, S. Li, B. Wei, H. Lv, L.-W. Chung, X. Zhang, Nat. Commun. 2018, 9, 2045.
- 3Selected examples:
- 3aS. Zhu, S. Yu, D. Ma, Angew. Chem. Int. Ed. 2008, 47, 545; Angew. Chem. 2008, 120, 555;
- 3bD. A. Nagib, M. E. Scott, D. W. C. MacMillan, J. Am. Chem. Soc. 2009, 131, 10875;
- 3cO. Lifchits, C. M. Reisinger, B. List, J. Am. Chem. Soc. 2010, 132, 10227;
- 3dX. Zhao, D. Liu, H. Guo, Y. Liu, W. Zhang, J. Am. Chem. Soc. 2011, 133, 19354;
- 3eJ. M. Stevens, D. W. C. MacMillan, J. Am. Chem. Soc. 2013, 135, 11756;
- 3fE. D. Nacsa, D. W. C. MacMillan, J. Am. Chem. Soc. 2018, 140, 3322.
- 4Selected examples:
- 4aT. Ooi, K. Doda, K. Maruoka, J. Am. Chem. Soc. 2003, 125, 9022;
- 4bJ.-F. Paquin, C. Defieber, C. R. J. Stephenson, E. M. Carreira, J. Am. Chem. Soc. 2005, 127, 10850;
- 4cM. Fañanás-Mastral, B. L. Feringa, J. Am. Chem. Soc. 2010, 132, 13152;
- 4dY. Gu, Y. Wang, T.-Y. Yu, Y.-M. Liang, P.-F. Xu, Angew. Chem. Int. Ed. 2014, 53, 14128; Angew. Chem. 2014, 126, 14352;
- 4eM. Silvi, C. Verrier, Y. P. Rey, L. Buzzetti, P. Melchiorre, Nat. Chem. 2017, 9, 868;
- 4fP. Bonilla, Y. P. Rey, C. M. Holden, P. Melchiorre, Angew. Chem. Int. Ed. 2018, 57, 12819; Angew. Chem. 2018, 130, 13001.
- 5Selected examples:
- 5aJ. W. Yang, M. T. H. Fonseca, N. Vignola, B. List, Angew. Chem. Int. Ed. 2005, 44, 108; Angew. Chem. 2005, 117, 110;
- 5bS. G. Ouellet, J. B. Tuttle, D. W. C. MacMillan, J. Am. Chem. Soc. 2005, 127, 32;
- 5cS. Mayer, B. List, Angew. Chem. Int. Ed. 2006, 45, 4193; Angew. Chem. 2006, 118, 4299;
- 5dN. Arai, K. Sato, K. Azuma, T. Ohkuma, Angew. Chem. Int. Ed. 2013, 52, 7500; Angew. Chem. 2013, 125, 7648;
- 5eT. M. Bräuer, Q. Zhang, K. Tiefenbacher, Angew. Chem. Int. Ed. 2016, 55, 7698; Angew. Chem. 2016, 128, 7829;
- 5fY. Zhou, J. S. Bandar, R. Y. Liu, S. L. Buchwald, J. Am. Chem. Soc. 2018, 140, 606.
- 6Reviews:
- 6aJ.-H. Xie, S.-F. Zhu, Q.-L. Zhou, Chem. Rev. 2011, 111, 1713;
- 6bD.-S. Wang, Q.-A. Chen, S.-M. Lu, Y.-G. Zhou, Chem. Rev. 2012, 112, 2557;
- 6cQ.-A. Chen, Z.-S. Ye, Y. Duan, Y.-G. Zhou, Chem. Soc. Rev. 2013, 42, 497;
- 6dJ. J. Verendel, O. Pàmies, M. Diéguez, P. G. Andersson, Chem. Rev. 2014, 114, 2130;
- 6eZ. Zhang, N. A. Butt, W. Zhang, Chem. Rev. 2016, 116, 14769;
- 6fZ. Zhang, N. A. Butt, M. Zhou, D. Liu, W. Zhang, Chin. J. Chem. 2018, 36, 443;
- 6gG. Xu, C. H. Senanayake, W. Tang, Acc. Chem. Res. 2019, 52, 1101; Recent examples:
- 6hM. R. Friedfeld, H. Zhong, R. T. Ruck, M. Shevlin, P. J. Chirik, Science 2018, 360, 888;
- 6iQ. Yan, G. Xiao, Y. Wang, G. Zi, Z. Zhang, G. Hou, J. Am. Chem. Soc. 2019, 141, 1749;
- 6jX. Li, J. Tian, N. Liu, X. Tu, N. Zeng, X. Wang, Angew. Chem. Int. Ed. 2019, 58, 4664; Angew. Chem. 2019, 131, 4712;
- 6kL. Zhang, Y. Tang, Z. Han, K. Ding, Angew. Chem. Int. Ed. 2019, 58, 4973; Angew. Chem. 2019, 131, 5027.
- 7Selected examples:
- 7aD. Zhao, B. Beiring, F. Glorius, Angew. Chem. Int. Ed. 2013, 52, 8454–8458; Angew. Chem. 2013, 125, 8612–8616;
- 7bX. Liu, Z. Han, Z. Wang, K. Ding, Angew. Chem. Int. Ed. 2014, 53, 1978; Angew. Chem. 2014, 126, 2009;
- 7cM. Bernasconi, M.-A. Müller, A. Pfaltz, Angew. Chem. Int. Ed. 2014, 53, 5385; Angew. Chem. 2014, 126, 5489;
- 7dW. Li, C. Schlepphorst, C. Daniliuc, F. Glorius, Angew. Chem. Int. Ed. 2016, 55, 3300; Angew. Chem. 2016, 128, 3361;
- 7eM. Shevlin, M. R. Friedfeld, H. Sheng, N. A. Pierson, J. M. Hoyt, L.-C. Campeau, P. J. Chirik, J. Am. Chem. Soc. 2016, 138, 3562;
- 7fM.-L. Li, S. Yang, X.-C. Su, H.-L. Wu, L.-L. Yang, S.-F. Zhu, Q.-L. Zhou, J. Am. Chem. Soc. 2017, 139, 541;
- 7gS. Ponra, J. Yang, S. Kerdphon, P. G. Andersson, Angew. Chem. Int. Ed. 2019, https://doi.org/10.1002/anie.201903954; Angew. Chem. 2019, https://doi.org/10.1002/ange.201903954.
- 8
- 8aT.-P. Dang, P. Aviron-Violet, Y. Colleuille, J. Varagnat, J. Mol. Catal. 1982, 16, 51;
- 8bS.-Y. Zhang, L.-F. Wang, M.-M. Gu, X. Gao, Chin. J. Org. Chem. 1991, 11, 306;
- 8cC. Chapuis, M. Barthe, J.-Y. de Saint Laumer, Helv. Chim. Acta 2001, 84, 230.
- 9Representative papers:
- 9aF. Tian, D. Yao, Y. Liu, F. Xie, W. Zhang, Adv. Synth. Catal. 2010, 352, 1841;
- 9bY. Liu, W. Zhang, Angew. Chem. Int. Ed. 2013, 52, 2203; Angew. Chem. 2013, 125, 2259;
- 9cJ. Chen, D. Liu, N. Butt. C. Li, D. Fan, Y. Liu, W. Zhang, Angew. Chem. Int. Ed. 2013, 52, 11632; Angew. Chem. 2013, 125, 11846;
- 9dY. Liu, I. D. Gridnev, W. Zhang, Angew. Chem. Int. Ed. 2014, 53, 1901; Angew. Chem. 2014, 126, 1932;
- 9eQ. Hu, Z. Zhang, Y. Liu, T. Imamoto, W. Zhang, Angew. Chem. Int. Ed. 2015, 54, 2260; Angew. Chem. 2015, 127, 2288;
- 9fZ. Zhang, Q. Hu, Y. Wang, J. Chen, W. Zhang, Org. Lett. 2015, 17, 5380;
- 9gQ. Hu, J. Chen, Z. Zhang, Y. Liu, W. Zhang, Org. Lett. 2016, 18, 1290;
- 9hJ. Chen, Z. Zhang, D. Liu, W. Zhang, Angew. Chem. Int. Ed. 2016, 55, 8444; Angew. Chem. 2016, 128, 8584;
- 9iQ. Hu, Y. Hu, Y. Liu, Z. Zhang, Y. Liu, W. Zhang, Chem. Eur. J. 2017, 23, 1040;
- 9jD. Fan, Y. Hu, F. Jiang, Z. Zhang, W. Zhang, Adv. Synth. Catal. 2018, 360, 2228;
- 9kC. Liu, J. Yuan, J. Zhang, Z. Wang, Z. Zhang, W. Zhang, Org. Lett. 2018, 20, 108;
- 9lJ. Zhang, C. Liu, X. Wang, J. Chen, Z. Zhang, W. Zhang, Chem. Commun. 2018, 54, 6024;
- 9mJ. Jia, Z. Ling, Z. Zhang, K. Tamura, I. D. Gridnev, T. Imamoto, W. Zhang, Adv. Synth. Catal. 2018, 360, 738;
- 9nJ. Jia, D. Fan, J. Zhang, Z. Zhang, W. Zhang, Adv. Synth. Catal. 2018, 360, 3793;
- 9oJ. Chen, Z. Zhang, B. Li, F. Li, Y. Wang, M. Zhao, I. D. Gridnev, T. Imamoto, W. Zhang, Nat. Commun. 2018, 9, 5000;
- 9pD. Fan, Y. Liu, J. Jia, Z. Zhang, Y. Liu, W. Zhang, Org. Lett. 2019, 21, 1042;
- 9qB. Li, J. Zhang, Z. Zhang, I. D. Gridnev, W. Zhang, Angew. Chem. Int. Ed. 2019, 58, 7329; Angew. Chem. 2019, 131, 7407.
- 10
- 10aF. Kopp, C. Mahlert, J. Grünewald, M. A. Marahiel, J. Am. Chem. Soc. 2006, 128, 16478;
- 10bA. Dixit, G. M. Verkhivker, J. Chem. Inf. Model. 2012, 52, 2501;
- 10cJ. Jerbi, M. Springborg, H. den-Haan, J. P. Cerón-Carrasco, Chem. Phys. Lett. 2017, 690, 74.
- 11
- 11aK. Tamura, M. Sugiya, K. Yoshida, A. Yanagisawa, T. Imamoto, Org. Lett. 2010, 12, 4400;
- 11bT. Imamoto, K. Tamura, Z. Zhang, Y. Horiuchi, M. Sugiya, K. Yoshida, A. Yanagisawa, I. D. Gridnev, J. Am. Chem. Soc. 2012, 134, 1754.
- 12The (R)-configuration of 2 w and 16 was assigned by X-ray crystallography. CCDC 1902245 and 1902246 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 13S. Müller, B. Liepold, G. J. Roth, H. J. Bestmann, Synlett 1996, 521.
- 14B. de Lange, D. J. Hyett, P. J. D. Maas, D. Mink, F. B. J. van Assema, N. Sereinig, A. H. M. de Vries, J. G. de Vries, ChemCatChem 2011, 3, 289.
- 15H. Zhao, X. He, A. Thurkauf, D. Hoffman, A. Kieltyka, R. Brodbeck, R. Primus, J. W. F. Wasley, Bioorg. Med. Chem. Lett. 2002, 12, 3111.
- 16S. Lee, K. Y. Yi, S.-e. Yoo, Bull. Korean Chem. Soc. 2004, 25, 207.
- 17
- 17aS. E. Denmark, N. Nakajima, C. M. Stiff, O. J.-C. Nicaise, M. Kranze, Adv. Synth. Catal. 2008, 350, 1023;
- 17bM. Azechi, K. Yamabuki, K. Onimura, T. Oishi, Polym. J. 2010, 42, 632.
- 18
- 18aI. D. Gridnev, T. Imamoto, Acc. Chem. Res. 2004, 37, 633;
- 18bI. D. Gridnev, T. Imamoto, Chem. Commun. 2009, 48, 7447;
- 18cI. D. Gridnev, P. A. Dub, Enantioselection in Asymmetric Catalysis, CRC, Boca Raton, FL, 2016, pp. 1–68.
10.1201/9781315370415-2 Google Scholar
- 19
- 19aI. D. Gridnev, N. Higashi, K. Asakura, T. Imamoto, J. Am. Chem. Soc. 2000, 122, 7183;
- 19bI. D. Gridnev, N. Higashi, T. Imamoto, J. Am. Chem. Soc. 2000, 122, 10486;
- 19cI. D. Gridnev, N. Higashi, T. Imamoto, J. Am. Chem. Soc. 2001, 123, 4631;
- 19dI. D. Gridnev, Y. Yamanoi, N. Higashi, H. Tsuruta, M. Yasutake, T. Imamoto, Adv. Synth. Catal. 2001, 343, 118;
- 19eM. Yasutake, I. D. Gridnev, N. Higashi, T. Imamoto, Org. Lett. 2001, 3, 1701;
- 19fI. D. Gridnev, N. Higashi, T. Imamoto, Organometallics 2001, 20, 4542;
- 19gI. D. Gridnev, M. Yasutake, N. Higashi, T. Imamoto, J. Am. Chem. Soc. 2001, 123, 5268;
- 19hI. D. Gridnev, M. Yasutake, T. Imamoto, I. P. Beletskaya, Proc. Natl. Acad. Sci. USA 2004, 101, 5385;
- 19iY. Wada, T. Imamoto, H. Tsuruta, K. Yamaguchi, I. D. Gridnev, Adv. Synth. Catal. 2004, 346, 777;
- 19jT. Imamoto, K. Yashio, K. V. L. Crépy, K. Katagiri, H. Takahashi, M. Kouchi, I. D. Gridnev, Organometallics 2006, 25, 908;
- 19kI. D. Gridnev, T. Imamoto, G. Hoge, M. Kouchi, H. Takahashi, J. Am. Chem. Soc. 2008, 130, 2560;
- 19lT. Imamoto, T. Itoh, K. Yoshida, I. D. Gridnev, Chem. Asian J. 2008, 3, 1636;
- 19mI. D. Gridnev, C. Kohrt, Y. Liu, Dalton Trans. 2014, 43, 1785;
- 19nI. D. Gridnev, Y. Liu, T. Imamoto, ACS Catal. 2014, 4, 203;
- 19oP. A. Dub, N. J. Henson, R. L. Martin, J. C. Gordon, J. Am. Chem. Soc. 2014, 136, 3505;
- 19pI. D. Gridnev, T. Imamoto, ACS Catal. 2015, 5, 2911;
- 19qI. D. Gridnev, T. Imamoto, Russ. Chem. Bull. 2016, 65, 1514;
- 19rI. D. Gridnev, ChemCatChem 2016, 8, 3463;
- 19sP. A. Dub, A. Matsunami, S. Kuwata, Y. Kayaki, J. Am. Chem. Soc. 2019, 141, 2661.