Switchable Reversible Addition–Fragmentation Chain Transfer (RAFT) Polymerization with the Assistance of Azobenzenes
Huijun Nie
Key Laboratory of Functional Polymer Materials of the Ministry of Education, Institute of Polymer Chemistry, College of Chemistry, Nankai University, 300071 Tianjin, China
These authors contributed equally to this work.
Search for more papers by this authorShenzhen Li
Key Laboratory of Functional Polymer Materials of the Ministry of Education, Institute of Polymer Chemistry, College of Chemistry, Nankai University, 300071 Tianjin, China
These authors contributed equally to this work.
Search for more papers by this authorSijia Qian
Key Laboratory of Functional Polymer Materials of the Ministry of Education, Institute of Polymer Chemistry, College of Chemistry, Nankai University, 300071 Tianjin, China
Search for more papers by this authorCorresponding Author
Dr. Zhongqiang Han
State Key Laboratory of Special Functional Waterproof Materials, Beijing Oriental Yuhong Waterproof Technology Co., Ltd., 100123 Beijing, China
Search for more papers by this authorCorresponding Author
Prof. Wangqing Zhang
Key Laboratory of Functional Polymer Materials of the Ministry of Education, Institute of Polymer Chemistry, College of Chemistry, Nankai University, 300071 Tianjin, China
Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, 300071 Tianjin, China
Search for more papers by this authorHuijun Nie
Key Laboratory of Functional Polymer Materials of the Ministry of Education, Institute of Polymer Chemistry, College of Chemistry, Nankai University, 300071 Tianjin, China
These authors contributed equally to this work.
Search for more papers by this authorShenzhen Li
Key Laboratory of Functional Polymer Materials of the Ministry of Education, Institute of Polymer Chemistry, College of Chemistry, Nankai University, 300071 Tianjin, China
These authors contributed equally to this work.
Search for more papers by this authorSijia Qian
Key Laboratory of Functional Polymer Materials of the Ministry of Education, Institute of Polymer Chemistry, College of Chemistry, Nankai University, 300071 Tianjin, China
Search for more papers by this authorCorresponding Author
Dr. Zhongqiang Han
State Key Laboratory of Special Functional Waterproof Materials, Beijing Oriental Yuhong Waterproof Technology Co., Ltd., 100123 Beijing, China
Search for more papers by this authorCorresponding Author
Prof. Wangqing Zhang
Key Laboratory of Functional Polymer Materials of the Ministry of Education, Institute of Polymer Chemistry, College of Chemistry, Nankai University, 300071 Tianjin, China
Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, 300071 Tianjin, China
Search for more papers by this authorGraphical Abstract
Abstract
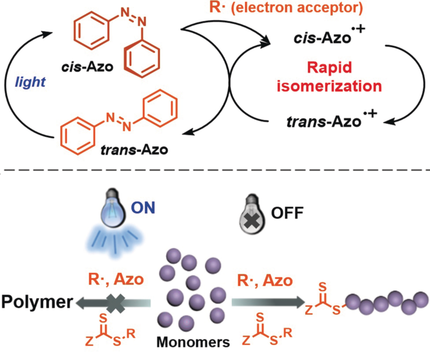
Modulating controlled radical polymerization is an interesting and important issue. Herein, modulating RAFT polymerization employing photosensitive azobenzenes is achieved. In the presence of azobenzenes and with visible light off, RAFT polymerization runs smoothly and follows a pseudo-first-order kinetics. In contrast, with light on, RAFT polymerization is greatly decelerated or quenched depending on the type and concentration of azobenzenes. Switchable RAFT polymerization of different (meth)acrylate monomers alternatively with light off and on is demonstrated. A mechanism of photoregulating RAFT polymerization involving radical quenching by azobenzenes is proposed.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201904991-sup-0001-misc_information.pdf2.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aG. Moad, E. Rizzardo, S. H. Thang, Acc. Chem. Res. 2008, 41, 1133–1142;
- 1bX. Pan, M. A. Tasdelen, J. Laun, T. Junkers, Y. Yagci, K. Matyjaszewski, Prog. Polym. Sci. 2016, 62, 73–125;
- 1cM. Chen, M. Zhong, J. A. Johnson, Chem. Rev. 2016, 116, 10167–10211;
- 1dM. Ouchi, T. Terashima, M. Sawamoto, Chem. Rev. 2009, 109, 4963–5050;
- 1eW. A. Braunecker, K. Matyjaszewski, Prog. Polym. Sci. 2007, 32, 93–146;
- 1fB. M. Rosen, V. Percec, Chem. Rev. 2009, 109, 5069–5119;
- 1gC. Lv, C. He, X. Pan, Angew. Chem. Int. Ed. 2018, 57, 9430–9433; Angew. Chem. 2018, 130, 9574–9577.
- 2
- 2aS. Yamago, Chem. Rev. 2009, 109, 5051–5068;
- 2bG. R. Jones, A. Anastasaki, R. Whitfield, N. Engelis, E. Liarou, D. M. Haddleton, Angew. Chem. Int. Ed. 2018, 57, 10468–10482; Angew. Chem. 2018, 130, 10628–10643;
- 2cA. Anastasaki, V. Nikolaou, G. Nurumbetov, P. Wilson, K. Kempe, J. F. Quinn, T. P. Davis, M. R. Whittaker, D. M. Haddleton, Chem. Rev. 2016, 116, 835–877;
- 2dC. J. Hawker, A. W. Bosman, E. Harth, Chem. Rev. 2001, 101, 3661–3688;
- 2eD. J. Keddie, Chem. Soc. Rev. 2014, 43, 496–505;
- 2fM. C. Iovu, K. Matyjaszewski, Macromolecules 2003, 36, 9346–9354.
- 3
- 3aM. R. Hill, R. N. Carmean, B. S. Sumerlin, Macromolecules 2015, 48, 5459–5469;
- 3bM. Uchiyama, K. Satoh, M. Kamigaito, Angew. Chem. Int. Ed. 2015, 54, 1924–1928; Angew. Chem. 2015, 127, 1944–1948;
- 3cA. Gregory, M. H. Stenzel, Prog. Polym. Sci. 2012, 37, 38–105;
- 3dT. G. McKenzie, Q. Fu, M. Uchiyama, K. Satoh, J. Xu, C. Boyer, M. Kamigaito, G. G. Qiao, Adv. Sci. 2016, 3, 1500394.
- 4
- 4aG. Moad, D. Keddie, C. Guerrero-Sanchez, E. Rizzardo, S. H. Thang, Macromol. Symp. 2015, 350, 34–42;
- 4bM. Benaglia, J. Chiefari, Y. K. Chong, G. Moad, E. Rizzardo, S. H. Thang, J. Am. Chem. Soc. 2009, 131, 6914–6915;
- 4cS. Shanmugam, J. Xu, C. Boyer, Angew. Chem. Int. Ed. 2016, 55, 1036–1040; Angew. Chem. 2016, 128, 1048–1052;
- 4dX. Pan, X. Guo, B. Choi, A. C. Feng, X. Wei, S. H. Thang, Polym. Chem. 2019, 10, 2083–2090;
- 4eS. J. Stace, C. M. Fellows, G. Moad, D. J. Keddie, Macromol. Rapid Commun. 2018, 39, 1800228.
- 5
- 5aS. Dadashi-Silab, S. Doran, Y. Yagci, Chem. Rev. 2016, 116, 10212–10275;
- 5bJ. P. Fouassier, X. Allonas, D. Burget, Prog. Org. Coat. 2003, 47, 16–36;
- 5cS. Yamago, Y. Nakamura, Polymer 2013, 54, 981–994;
- 5dA. Wolpers, P. Vana, Macromolecules 2014, 47, 954–963;
- 5eC. Ding, C. Fan, G. Jiang, X. Pan, Z. Zhang, J. Zhu, X. Zhu, Macromol. Rapid Commun. 2015, 36, 2181–2185.
- 6
- 6aT. Otsu, J. Polym. Sci. Part A 2000, 38, 2121–2136;
10.1002/(SICI)1099-0518(20000615)38:12<2121::AID-POLA10>3.0.CO;2-X CAS Web of Science® Google Scholar
- 6bM. Y. Khan, M. S. Cho, Y. J. Kwark, Macromolecules 2014, 47, 1929–1934;
- 6cK. Jung, C. Boyer, P. B. Zetterlund, Polym. Chem. 2017, 8, 3965–3970.
- 7
- 7aY. Yagci, S. Jockusch, N. J. Turro, Macromolecules 2010, 43, 6245–6260;
- 7bJ. He, D. Liu, J. Tan, L. Zhang, Polymer 2018, 145, 70–79;
- 7cJ. Tan, Y. Bai, X. Zhang, C. Huang, D. Liu, L. Zhang, Macromol. Rapid Commun. 2016, 37, 1434–1440.
- 8
- 8aH. Gong, Y. Zhao, X. Shen, J. Lin, M. Chen, Angew. Chem. Int. Ed. 2018, 57, 333–337; Angew. Chem. 2018, 130, 339–343;
- 8bJ. Phommalysack-Lovan, Y. Chu, C. Boyer, J. Xu, Chem. Commun. 2018, 54, 6591–6606;
- 8cJ. C. Theriot, G. M. Miyake, C. Boyer, ACS Macro Lett. 2018, 7, 662–666;
- 8dJ. Jiang, G. Ye, Z. Wang, Y. Lu, J. Chen, K. Matyjaszewski, Angew. Chem. Int. Ed. 2018, 57, 12037–12042; Angew. Chem. 2018, 130, 12213–12218;
- 8eK. Tu, T. Xu, L. Zhang, Z. Cheng, X. Zhu, RSC Adv. 2017, 7, 24040–24045.
- 9
- 9aM. A. Tasdelen, Y. Y. Durmaz, B. Karagoz, N. Bicak, Y. Yagci, J. Polym. Sci. Part A 2008, 46, 3387–3395;
- 9bR. Francis, A. Ajayaghosh, Macromolecules 2000, 33, 4699–4704.
- 10
- 10aS. Muthukrishnan, E. H. Pan, M. H. Stenzel, C. Barner-Kowollik, T. P. Davis, D. Lewis, L. Barner, Macromolecules 2007, 40, 2978–2980;
- 10bH. Zhang, J. Deng, L. Lu, Y. Cai, Macromolecules 2007, 40, 9252–9261;
- 10cA. Ohtsuki, L. Lei, M. Tanishima, A. Goto, H. Kaji, J. Am. Chem. Soc. 2015, 137, 5610–5617.
- 11
- 11aJ. Xu, S. Shanmugam, H. T. Duong, C. Boyer, Polym. Chem. 2015, 6, 5615–5624;
- 11bC. Wu, N. Corrigan, C. H. Lim, K. Jung, J. Zhu, G. Miyake, J. Xu, C. Boyer, Macromolecules 2019, 52, 236–248.
- 12
- 12aA. A. Beharry, G. A. Woolley, Chem. Soc. Rev. 2011, 40, 4422–4437;
- 12bZ. F. Liu, K. Hashimoto, A. Fujishima, Nature 1990, 347, 658–660;
- 12cJ. Zielonka, R. Podsiadły, M. Czerwińska, A. Sikora, J. Sokołowska, A. Marcinek, J. Photochem. Photobiol. A 2004, 163, 373–379;
- 12dE. Katz, Electroanalysis 2018, 30, 759–797;
- 12eT. Schultz, J. Quenneville, B. Levine, A. Toniolo, T. J. Martínez, S. Lochbrunner, M. Schmitt, J. P. Shaffer, M. Z. Zgierski, A. Stolow, J. Am. Chem. Soc. 2003, 125, 8098–8099.
- 13
- 13aP. Bortolus, S. Monti, A. Albini, E. Fasani, S. Pietra, J. Org. Chem. 1989, 54, 534–540;
- 13bF. M. Raymo, M. Tomasulo, Chem. Soc. Rev. 2005, 34, 327–336;
- 13cJ. O. Morley, O. J. Guy, M. H. Charlton, J. Photochem. Photobiol. A 2005, 173, 174–184;
- 13dR. Podsiadły, J. Sokołowska, Color. Technol. 2003, 119, 341–344;
- 13eS. C. Yan, Z. S. Li, Z. G. Zou, Langmuir 2010, 26, 3894–3901.
- 14
- 14aL. Flamigni, S. Monti, J. Phys. Chem. 1985, 89, 3702–3707;
- 14bD. G. Lishan, P. W. Harris, K. Brahim, R. L. Jackson, J. Soc. Dyers Colour. 1988, 104, 33–37.
- 15
- 15aA. Albini, E. Fasani, S. Pietra, J. Chem. Soc. Perkin Trans. 2 1982, 1393–1395;
- 15bA. Albini, E. Fasani, M. Moroni, S. Pietra, J. Chem. Soc. Perkin Trans. 2 1986, 1439–1443.
- 16C. Zhang, M. H. Du, H. P. Cheng, X. G. Zhang, A. E. Roitberg, J. L. Krause, Phys. Rev. Lett. 2004, 92, 158301.
- 17
- 17aA. Goulet-Hanssens, M. Utecht, D. Mutruc, E. Titov, J. Schwarz, L. Grubert, D. Bléger, P. Saalfrank, S. Hecht, J. Am. Chem. Soc. 2017, 139, 335–341;
- 17bA. Goulet-Hanssens, C. Rietze, E. Titov, L. Abdullahu, L. Grubert, P. Saalfrank, S. Hecht, Chem 2018, 4, 1740–1755.
- 18Y. You, W. Nam, Chem. Soc. Rev. 2012, 41, 7061–7084.
- 19N. A. Romero, D. A. Nicewicz, Chem. Rev. 2016, 116, 10075–10166.
- 20
- 20aG. L. Li, J. Q. Liu, B. D. Zhao, T. Wang, Spectrochim. Acta Part A 2013, 104, 287–291;
- 20bM. Bouchouit, Y. Elkouari, L. Messaadia, A. Bouraiou, S. Arroudj, S. Bouacida, S. Taboukhat, K. Bouchouit, Opt. Quantum Electron. 2016, 48, 178;
- 20cM. Staniszewska, S. Kupfer, M. Łabuda, J. Guthmuller, J. Chem. Theory Comput. 2017, 13, 1263–1274.
- 21
- 21aW. Zhao, W. Bian, J. Mol. Struct. THEOCHEM 2007, 818, 43–49;
- 21bK. Munkerup, D. Romanov, T. Bohinski, A. B. Stephansen, R. J. Levis, T. I. Sølling, J. Phys. Chem. A 2017, 121, 8642–8651.
- 22T. Cusati, G. Granucci, M. Persico, G. Spighi, J. Chem. Phys. 2008, 128, 194312.
Citing Literature
August 12, 2019
Pages 11449-11453