Phosphanyl Cyanophosphide Salts: Versatile PCN Building Blocks
Dr. Zhongshu Li
Lehn Institute of Functional Materials (LIFM), School of Chemistry, Sun Yat-Sen University, 510275 Guangzhou, China
State Key Laboratory of Elemento-Organic Chemistry, Nankai University, 30071 Tianjin, China
Search for more papers by this authorCorresponding Author
Dr. Jaap E. Borger
Department of Chemistry and Applied Biosciences, ETH Zürich, Vladimir-Prelog-Weg 1, 8093 Zürich, Switzerland
Search for more papers by this authorFabian Müller
Department of Chemistry and Applied Biosciences, ETH Zürich, Vladimir-Prelog-Weg 1, 8093 Zürich, Switzerland
Search for more papers by this authorProf. Dr. Jeffrey R. Harmer
Centre for Advanced Imaging, University of Queensland, Brisbane, QLD, 4072 Australia
Search for more papers by this authorProf. Dr. Cheng-Yong Su
Lehn Institute of Functional Materials (LIFM), School of Chemistry, Sun Yat-Sen University, 510275 Guangzhou, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Hansjörg Grützmacher
Lehn Institute of Functional Materials (LIFM), School of Chemistry, Sun Yat-Sen University, 510275 Guangzhou, China
Department of Chemistry and Applied Biosciences, ETH Zürich, Vladimir-Prelog-Weg 1, 8093 Zürich, Switzerland
Search for more papers by this authorDr. Zhongshu Li
Lehn Institute of Functional Materials (LIFM), School of Chemistry, Sun Yat-Sen University, 510275 Guangzhou, China
State Key Laboratory of Elemento-Organic Chemistry, Nankai University, 30071 Tianjin, China
Search for more papers by this authorCorresponding Author
Dr. Jaap E. Borger
Department of Chemistry and Applied Biosciences, ETH Zürich, Vladimir-Prelog-Weg 1, 8093 Zürich, Switzerland
Search for more papers by this authorFabian Müller
Department of Chemistry and Applied Biosciences, ETH Zürich, Vladimir-Prelog-Weg 1, 8093 Zürich, Switzerland
Search for more papers by this authorProf. Dr. Jeffrey R. Harmer
Centre for Advanced Imaging, University of Queensland, Brisbane, QLD, 4072 Australia
Search for more papers by this authorProf. Dr. Cheng-Yong Su
Lehn Institute of Functional Materials (LIFM), School of Chemistry, Sun Yat-Sen University, 510275 Guangzhou, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Hansjörg Grützmacher
Lehn Institute of Functional Materials (LIFM), School of Chemistry, Sun Yat-Sen University, 510275 Guangzhou, China
Department of Chemistry and Applied Biosciences, ETH Zürich, Vladimir-Prelog-Weg 1, 8093 Zürich, Switzerland
Search for more papers by this authorGraphical Abstract
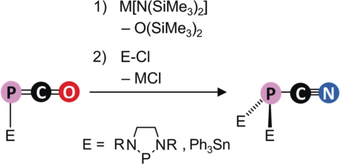
A new brick: The oxygen center in the phosphaketenes E-P=C=O can be readily replaced by nitrogen to give alkali salts of phosphanyl cyanophosphides [E-PCN]−, which are further converted into E2(PCN). These compounds can be used as synthons for the dianion [PCN]2−, which is a building block for PCNB heteroallenes and PCN-containing metal complexes.
Abstract
The facile preparation of alkali salts of phosphanyl cyanophosphides [NHP-PCN]− (NHP=N-heterocyclic phosphenium) is reported. Their formation is achieved by isoelectronic replacement of O for [N]− in the phosphaketenes NHP-PCO using alkaline hexamethyldisilazide M[N(SiMe3)2] (M=Na, K) as reagent. The new anionic entities are versatile PCN building blocks which allow the formation of a diversity of new cyanophosphine derivates including the first example of a PCNB hetero-cumulene and a PCN-ligated transition metal complex.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201904720-sup-0001-misc_information.pdf1.9 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. E. House, Inorganic Chemistry, 2nd ed., Academic Press, New York, 2013, pp. 393–437;
10.1016/B978-0-12-385110-9.00013-3 Google Scholar
- 1b“Cyanamides”: T. Güthner, B. Mertschenk in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2006, https://doi.org/10.1002/14356007.a08_139.pub2.
- 2For reviews on cyanamide chemistry, see:
- 2aM. R. R. Prabhath, L. Williams, S. V. Bhat, P. Sharma, Molecules 2017, 22, 615;
- 2bM.-H. Larraufie, G. Maestri, M. Malacria, C. Ollivier, L. Fensterbank, E. Lacôte, Synthesis 2012, 44, 1279–1292;
- 2cD. D. Nekrasov, Russ. J. Org. Chem. 2004, 40, 1387–1402.
- 3In one report cyanophosphandiide was evaluated computationally, see: P. Pyykkö, Y. Zhao, Mol. Phys. 1990, 70, 701–714.
- 4For a theoretical study of neutral isomers PCN and PNC, see: A. Largo, C. Barrientos, J. Phys. Chem. 1991, 95, 9864–9868.
- 5
- 5aA. Schmidpeter, F. Zwaschka, Angew. Chem. Int. Ed. Engl. 1977, 16, 704–705; Angew. Chem. 1977, 89, 747–747;
- 5bA. Schmidpeter, F. Zwaschka, W. S. Sheldrick, Chem. Ber. 1985, 118, 1078–1085.
- 6
- 6aA. Schmidpeter, G. Burget, F. Zwaschka, W. S. Sheldrick, Z. Anorg. Allg. Chem. 1985, 527, 17–32;
- 6bA. Schmidpeter, G. Burget, D. J. Chandler, R. A. Jones, Inorg. Synth. 2007, 126–129.
10.1002/9780470132562.ch29 Google Scholar
- 7Recently, Macdonald and co-workers likewise reported a method to prepare M+[P(CN)2]−, see: J. F. Binder, S. C. Kosnik, P. B. J. St Onge, C. L. B. Macdonald, Chem. Eur. J. 2018, 24, 14644–14648.
- 8For example, the synthesis of P(CN)3 was achieved for the first time by Gall and Schüppen using AgCN and PCl3, see: H. Gall, J. Schüppen, Ber. Dtsch. Chem. Ges. B 1930, 63, 482–487.
10.1002/cber.19300630237 Google Scholar
- 9A. Schmidpeter, K.-H. Zirzow, G. Burget, G. Huttner, I. Jibril, Chem. Ber. 1984, 117, 1695–1706.
- 10R. M. K. Deng, K. B. Dillon, J. Chem. Soc. Chem. Commun. 1981, 1170–1171.
- 11A. Schmidpeter, G. Bürget, Z. Naturforsch. B 1985, 40, 1306–1313.
- 12For reviews, see:
- 12aH. Grützmacher, J. Goicoechea, Angew. Chem. Int. Ed. 2018, 57, 16968–16994; Angew. Chem. 2018, 130, 17214–17240;
- 12bL. Weber, Eur. J. Inorg. Chem. 2018, 2175–2227.
- 13Z. Li, X. Chen, M. Bergeler, M. Reiher, C.-Y. Su, H. Grützmacher, Dalton Trans. 2015, 44, 6431–6438.
- 14Diazaphospholidine phosphaketenes, containing a saturated backbone, have also been isolated, see:
- 14aZ. Li, X. Chen, Z. Benkő, L. Liu, D. A. Ruiz, J. L. Peltier, G. Bertrand, C.-Y. Su, H. Grützmacher, Angew. Chem. Int. Ed. 2016, 55, 6018–6022; Angew. Chem. 2016, 128, 6122–6126;
- 14bL. Liu, D. A. Ruiz, D. Munz, G. Bertrand, Chem 2016, 1, 147–153;
- 14cM. M. Hansmann, R. Jazzar, G. Bertrand, J. Am. Chem. Soc. 2016, 138, 8356–8359.
- 15
- 15aD. Gudat, A. Haghverdi, M. Nieger, Angew. Chem. Int. Ed. 2000, 39, 3084–3086;
10.1002/1521-3773(20000901)39:17<3084::AID-ANIE3084>3.0.CO;2-R CAS PubMed Web of Science® Google ScholarAngew. Chem. 2000, 112, 3211–3214;
- 15bS. Burck, D. Gudat, M. Nieger, Angew. Chem. Int. Ed. 2004, 43, 4801–4804; Angew. Chem. 2004, 116, 4905–4908;
- 15cS. Burck, D. Gudat, M. Nieger, W.-W. Du Mont, J. Am. Chem. Soc. 2006, 128, 3946–3955;
- 15dS. Burck, K. Götz, M. Kaupp, M. Nieger, J. Weber, J. Schmedt auf der Günne, D. Gudat, J. Am. Chem. Soc. 2009, 131, 10763–10774;
- 15eD. Gudat, Dalton Trans. 2016, 45, 5896–5907;
- 15fJ. Nickolaus, D. A. Imbrich, S. H. Schlindwein, A. H. Geyer, M. Nieger, D. Gudat, Inorg. Chem. 2017, 56, 3071–3080;
- 15gM. Papke, L. Dettling, J. A. W. Sklorz, D. Szieberth, L. Nyulászi, C. Müller, Angew. Chem. Int. Ed. 2017, 56, 16484–16489; Angew. Chem. 2017, 129, 16706–16712.
- 16
- 16aU. Wannagat, H. Steyffert, Angew. Chem. Int. Ed. Engl. 1965, 4, 438–439; Angew. Chem. 1965, 77, 457–458;
- 16bR. B. King, Inorg. Chem. 1967, 6, 25–29;
- 16cM. Moll, H. Behrens, P. Merbach, K.-H. Trummer, G. Thiele, K. Wittmann, Z. Naturforsch. B 1986, 41, 606–616.
- 17See the Supporting Information for details.
- 18L. Weber, Chem. Rev. 1992, 92, 1839–1906.
- 19
- 19aP. Pyykkö, M. Atsumi, Chem. Eur. J. 2009, 15, 186–197;
- 19bP. Pyykkö, M. Atsumi, Chem. Eur. J. 2009, 15, 12770–12779.
- 20T. L. Breen, D. W. Stephan, J. Am. Chem. Soc. 1995, 117, 11914–11921.
- 21The data also correspond well with the ones reported of a similar example synthesized from [P(CN)2]−, see: A. Schmidpeter, W. Gebler, F. Zwaschka, W. S. Sheldrick, Angew. Chem. Int. Ed. Engl. 1980, 19, 722–723; Angew. Chem. 1980, 92, 767–768; Angew. Chem. 1980, 92, 767–768.
- 22For a review, see:
- 22aT. Krachko, J. C. Slootweg, Eur. J. Inorg. Chem. 2018, 2734–2754;
- 22bK. Schwedtmann, G. Zanoni, J. J. Weigand, Chem. Eur. J. 2018, 24, 1388–1405;
- 22cA. Doddi, M. Peters, M. Tamm, Chem. Rev. 2019, https://doi.org/10.1021/acs.chemrev.8b00791.
- 23O. Puntigam, D. Förster, N. A. Giffin, S. Burck, J. Bender, F. Ehret, A. D. Hendsbee, M. Nieger, J. D. Masuda, D. Gudat, Eur. J. Inorg. Chem. 2013, 2041–2050.
- 24S. Alvarez, Dalton Trans. 2013, 42, 8617–8636.
- 25C. C. Cummins, C. Huang, T. J. Miller, M. W. Reintinger, J. M. Stauber, I. Tannou, D. Tofan, A. Toubaei, A. Velian, G. Wu, Inorg. Chem. 2014, 53, 3678–3687.
- 26S. Haber, P. L. Floch, F. Mathey, J. Chem. Soc. Chem. Commun. 1992, 1799–1800.
- 27For a recent computational study of cyanophosphines, see: G. Sánchez-Sanz, C. Trujillo, I. Alkorta, J. Elguero, Comput. Theor. Chem. 2015, 1053, 305–314.
- 28For examples, see:
- 28aH. Schmidbaur, G. Weidenhiller, O. Steigelmann, Angew. Chem. Int. Ed. Engl. 1991, 30, 433–435; Angew. Chem. 1991, 103, 442–444;
- 28bS. A. Reiter, S. D. Nogai, H. Schmidbaur, Z. Naturforsch. B 2014, 60, 511–519.
10.1515/znb-2005-0506 Google Scholar