C−H and C−F Bond Activation Reactions of Fluorinated Propenes at Rhodium: Distinctive Reactivity of the Refrigerant HFO-1234yf
Dr. Maria Talavera
Department of Chemistry, Humboldt Universität zu Berlin, Brook-Taylor-Strasse 2, 12489 Berlin, Germany
These authors contributed equally to this work.
Search for more papers by this authorCortney N. von Hahmann
Department of Chemistry, Humboldt Universität zu Berlin, Brook-Taylor-Strasse 2, 12489 Berlin, Germany
These authors contributed equally to this work.
Search for more papers by this authorDr. Robert Müller
Institut für Chemie, Theoretische Chemie/Quantenchemie, Sekr. C7, Technische Universität Berlin, Strasse des 17. Juni 135, 10623 Berlin, Germany
Search for more papers by this authorDr. Mike Ahrens
Department of Chemistry, Humboldt Universität zu Berlin, Brook-Taylor-Strasse 2, 12489 Berlin, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Martin Kaupp
Institut für Chemie, Theoretische Chemie/Quantenchemie, Sekr. C7, Technische Universität Berlin, Strasse des 17. Juni 135, 10623 Berlin, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Thomas Braun
Department of Chemistry, Humboldt Universität zu Berlin, Brook-Taylor-Strasse 2, 12489 Berlin, Germany
Search for more papers by this authorDr. Maria Talavera
Department of Chemistry, Humboldt Universität zu Berlin, Brook-Taylor-Strasse 2, 12489 Berlin, Germany
These authors contributed equally to this work.
Search for more papers by this authorCortney N. von Hahmann
Department of Chemistry, Humboldt Universität zu Berlin, Brook-Taylor-Strasse 2, 12489 Berlin, Germany
These authors contributed equally to this work.
Search for more papers by this authorDr. Robert Müller
Institut für Chemie, Theoretische Chemie/Quantenchemie, Sekr. C7, Technische Universität Berlin, Strasse des 17. Juni 135, 10623 Berlin, Germany
Search for more papers by this authorDr. Mike Ahrens
Department of Chemistry, Humboldt Universität zu Berlin, Brook-Taylor-Strasse 2, 12489 Berlin, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Martin Kaupp
Institut für Chemie, Theoretische Chemie/Quantenchemie, Sekr. C7, Technische Universität Berlin, Strasse des 17. Juni 135, 10623 Berlin, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Thomas Braun
Department of Chemistry, Humboldt Universität zu Berlin, Brook-Taylor-Strasse 2, 12489 Berlin, Germany
Search for more papers by this authorDedicated to Professor Helmut Werner on the occasion of his 85th birthday
Graphical Abstract
Abstract
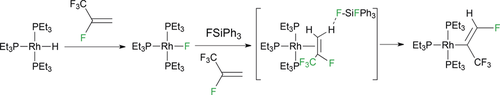
The reaction of [Rh(H)(PEt3)3] (1) with the refrigerant HFO-1234yf (2,3,3,3-tetrafluoropropene) affords an efficient route to obtain [Rh(F)(PEt3)3] (3) by C−F bond activation. Catalytic hydrodefluorinations were achieved in the presence of the silane HSiPh3. In the presence of a fluorosilane, 3 provides a C−H bond activation followed by a 1,2-fluorine shift to produce [Rh{(E)-C(CF3)=CHF}(PEt3)3] (4). Similar rearrangements of HFO-1234yf were observed at [Rh(E)(PEt3)3] [E=Bpin (6), C7D7 (8), Me (9)]. The ability to favor C−H bond activation using 3 and fluorosilane is also demonstrated with 3,3,3-trifluoropropene. Studies are supported by DFT calculations.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201902872-sup-0001-misc_information.pdf3.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aY. Zhou, J. Wang, Z. Gu, S. Wang, W. Zhu, J. L. Aceña, V. A. Soloshonok, K. Izawa, H. Liu, Chem. Rev. 2016, 116, 422–518;
- 1bS. Purser, P. R. Moore, S. Swallow, V. Gouverneur, Chem. Soc. Rev. 2008, 37, 320–330;
- 1cP. Jeschke, ChemBioChem 2004, 5, 570–589;
- 1dP. Kirsch, Modern Fluoroorganic Chemistry: Synthesis Reactivity and Applications, 2nd ed., Wiley-VCH, Weinheim, 2013.
10.1002/9783527651351 Google Scholar
- 2S. E. Manahan, Fundamentals of Environmental and Toxicological Chemistry, Vol. Fourth, CRC Press: Taylor & Francis Group, Boca Raton, FL, 2013.
10.1201/b13851 Google Scholar
- 3
- 3aN. O. Andrella, K. Liu, B. Gabidullin, M. Vasiliu, D. A. Dixon, R. T. Baker, Organometallics 2018, 37, 422–432;
- 3bO. Eisenstein, J. Milani, R. N. Perutz, Chem. Rev. 2017, 117, 8710–8753;
- 3cT. Ahrens, J. Kohlmann, M. Ahrens, T. Braun, Chem. Rev. 2015, 115, 931–972;
- 3dH. Amii, K. Uneyama, Chem. Rev. 2009, 109, 2119–2183;
- 3eK. Kikushima, H. Sakaguchi, H. Saijo, M. Ohashi, S. Ogoshi, Chem. Lett. 2015, 44, 1019–1021;
- 3fL. M. Alluhaibi, A. K. Brisdon, R. G. Pritchard, J. Fluorine Chem. 2017, 203, 146–154;
- 3gD. J. Harrison, G. M. Lee, M. C. Leclerc, I. Korobkov, R. T. Baker, J. Am. Chem. Soc. 2013, 135, 18296–18299;
- 3hM. E. Slaney, M. J. Ferguson, R. McDonald, M. Cowie, Organometallics 2012, 31, 1384–1396.
- 4
- 4aL. Keyes, J. A. Love, C−H and C-X Bond Functionalization: Transition Metal Mediation, The Royal Society of Chemistry, London, 2013;
- 4bM. K. Whittlesey, E. Peris, ACS Catal. 2014, 4, 3152–3159;
- 4cT. Fujita, K. Fuchibe, J. Ichikawa, Angew. Chem. Int. Ed. 2019, 58, 390–402; Angew. Chem. 2019, 131, 396–408;
- 4dW. Chen, C. Bakewell, M. R. Crimmin, Synthesis 2017, 49, 810–821.
- 5
- 5aG. Meier, T. Braun, Angew. Chem. Int. Ed. 2009, 48, 1546–1548; Angew. Chem. 2009, 121, 1575–1577;
- 5bR. J. Lindup, T. B. Marder, R. N. Perutz, A. C. Whitwood, Chem. Commun. 2007, 3664–3666;
- 5cM. Aizenberg, D. Milstein, Science 1994, 265, 359–361;
- 5dT. Stahl, H. F. T. Klare, M. Oestreich, ACS Catal. 2013, 3, 1578–1587;
- 5eM. F. Kuehnel, D. Lentz, T. Braun, Angew. Chem. Int. Ed. 2013, 52, 3328–3348; Angew. Chem. 2013, 125, 3412–3433.
- 6
- 6aD. Noveski, T. Braun, M. Schulte, B. Neumann, H.-G. Stammler, Dalton Trans. 2003, 4075–4083;
- 6bT. Braun, D. Noveski, B. Neumann, H.-G. Stammler, Angew. Chem. Int. Ed. 2002, 41, 2745–2748;
10.1002/1521-3773(20020802)41:15<2745::AID-ANIE2745>3.0.CO;2-C CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 2870–2873;
- 6cM. Teltewskoi, J. A. Panetier, S. A. Macgregor, T. Braun, Angew. Chem. Int. Ed. 2010, 49, 3947–3951; Angew. Chem. 2010, 122, 4039–4043;
- 6dT. Braun, M. Ahijado Salomon, K. Altenhöner, M. Teltewskoi, S. Hinze, Angew. Chem. Int. Ed. 2009, 48, 1818–1822; Angew. Chem. 2009, 121, 1850–1854;
- 6eT. Ahrens, M. Ahrens, T. Braun, B. Braun, R. Herrmann, Dalton Trans. 2016, 45, 4716–4728;
- 6fA. Lena Raza, M. F. Kuehnel, M. Talavera, M. Teltewskoi, M. Ahrens, P. Kläring, T. Braun, D. Lentz, J. Fluorine Chem. 2018, 214, 80–85.
- 7T. Ahrens, M. Teltewskoi, M. Ahrens, T. Braun, R. Laubenstein, Dalton Trans. 2016, 45, 17495–17507.
- 8
- 8aD. Noveski, T. Braun, B. Neumann, A. Stammler, H.-G. Stammler, Dalton Trans. 2004, 4106–4119;
- 8bS. I. Kalläne, M. Teltewskoi, T. Braun, B. Braun, Organometallics 2015, 34, 1156–1169;
- 8cA. L. Raza, J. A. Panetier, M. Teltewskoi, S. A. Macgregor, T. Braun, Organometallics 2013, 32, 3795–3807.
- 9E. Clot, O. Eisenstein, N. Jasim, S. A. Macgregor, J. E. McGrady, R. N. Perutz, Acc. Chem. Res. 2011, 44, 333–348.
- 10B. Minor, M. Spatz, HFO-1234yf Low GWP Refrigerant Update, International Refrigeration and Air Conditioning Conference, Paper 937, 2008.
- 11
- 11aW. Mao, Y. Bai, W. Wang, B. Wang, Q. Xu, L. Shi, C. Li, J. Lu, ChemCatChem 2017, 9, 824–832;
- 11bY. Hiraoka, T. Kawasaki-Takasuka, Y. Morizawa, T. Yamazaki, J. Fluorine Chem. 2015, 179, 71–76;
- 11cY. Li, D.-H. Tu, Y.-J. Gu, B. Wang, Y.-Y. Wang, Z.-T. Liu, Z.-W. Liu, J. Lu, Eur. J. Org. Chem. 2015, 4340–4343;
- 11dY. L. Yagupolskii, N. V. Pavlenko, S. V. Shelyazhenko, A. A. Filatov, M. M. Kremlev, A. I. Mushta, I. I. Gerus, S. Peng, V. A. Petrov, M. Nappa, J. Fluorine Chem. 2015, 179, 134–141;
- 11eH. Sakaguchi, Y. Uetake, M. Ohashi, T. Niwa, S. Ogoshi, T. Hosoya, J. Am. Chem. Soc. 2017, 139, 12855–12862;
- 11fG. Meißner, K. Kretschmar, T. Braun, E. Kemnitz, Angew. Chem. Int. Ed. 2017, 56, 16338–16341; Angew. Chem. 2017, 129, 16556–16559;
- 11gH. Sakaguchi, M. Ohashi, S. Ogoshi, Angew. Chem. Int. Ed. 2018, 57, 328–332; Angew. Chem. 2018, 130, 334–338;
- 11hC. Bakewell, A. White, M. R. Crimmin, Angew. Chem. Int. Ed. 2018, 57, 6638–6642; Angew. Chem. 2018, 130, 6748–6752.
- 12D. Noveski, T. Braun, S. Krückemeier, J. Fluorine Chem. 2004, 125, 959–966.
- 13R. Dorta, E. D. Stevens, N. M. Scott, C. Costabile, L. Cavallo, C. D. Hoff, S. P. Nolan, J. Am. Chem. Soc. 2005, 127, 2485–2495.
- 14
- 14aM. F. Kuehnel, P. Holstein, M. Kliche, J. Krüger, S. Matthies, D. Nitsch, J. Schutt, M. Sparenberg, D. Lentz, Chem. Eur. J. 2012, 18, 10701–10714;
- 14bB. M. Kraft, E. Clot, O. Eisenstein, W. W. Brennessel, W. D. Jones, J. Fluorine Chem. 2010, 131, 1122–1132;
- 14cR. T. Thornbury, F. D. Toste, Angew. Chem. Int. Ed. 2016, 55, 11629–11632; Angew. Chem. 2016, 128, 11801–11804;
- 14dT. Ichitsuka, T. Fujita, J. Ichikawa, ACS Catal. 2015, 5, 5947–5950;
- 14eJ. Hu, X. Han, Y. Yuan, Z. Shi, Angew. Chem. Int. Ed. 2017, 56, 13342–13346; Angew. Chem. 2017, 129, 13527–13531.
- 15
- 15aR. Kojima, K. Kubota, H. Ito, Chem. Commun. 2017, 53, 10688–10691, and references therein;
- 15bM. Ohashi, S. Ogoshi in Organometallic Fluorine Chemistry, Vol. 52 (Eds.: ), Springer, Cham, 2014.
- 16T. Braun, F. Wehmeier, K. Altenhöner, Angew. Chem. Int. Ed. 2007, 46, 5321–5324; Angew. Chem. 2007, 119, 5415–5418.
- 17L. Zámostná, T. Braun, Angew. Chem. Int. Ed. 2015, 54, 10652–10656; Angew. Chem. 2015, 127, 10798–10802.
- 18Note that full conversion of the rhodium precursor was achieved after the given reaction time according to the NMR data.
- 19Formation of phosphine and 5 can be explained by a dissociation of PEt3 from a cationic intermediate which resembles A (Scheme 5) leading in toluene as solvent to the cationic diphosphine rhodium complex [Rh(η6-C7D8)(PEt3)2]+. In addition, presence of a small amount of an unidentified precipitate suggests the formation of rhodium compounds without phosphine ligands.
- 20A. L. Raza, T. Braun, Chem. Sci. 2015, 6, 4255–4260.
- 21
- 21aN. L. Dean, J. S. McIndoe, Can. J. Chem. 2018, 96, 587–590;
- 21bK. Kikushima, M. Grellier, M. Ohashi, S. Ogoshi, Angew. Chem. Int. Ed. 2017, 56, 16191–16196; Angew. Chem. 2017, 129, 16409–16414.
- 22
- 22aJ. Horstmann, M. Niemann, K. Berthold, A. Mix, B. Neumann, H.-G. Stammler, N. W. Mitzel, Dalton Trans. 2017, 46, 1898–1913;
- 22bK. Tamao, T. Hayashi, Y. Ito, M. Shiro, Organometallics 1992, 11, 2099–2114.
- 23M. Crimmin, A. J. P. White, N. A. Phillips, Adv. Synth. Catal. 2019, https://doi.org/10.1002/adsc.201900234.
- 24M. Gómez-Gallego, M. A. Sierra, Chem. Rev. 2011, 111, 4857–4963.
- 25M. Pait, G. Kundu, S. Tothadi, S. Karak, S. Jain, K. Vanka, S. S. Sen, Angew. Chem. Int. Ed. 2019, 58, 2804–2808; Angew. Chem. 2019, 131, 2830–2834.
- 26
- 26aS. G. Curto, M. A. Esteruelas, M. Oliván, E. Oñate, A. Vélez, Organometallics 2018, 37, 1970–1978;
- 26bA. Guthertz, M. Leutzsch, L. M. Wolf, P. Gupta, S. M. Rummelt, R. Goddard, C. Farès, W. Thiel, A. Fürstner, J. Am. Chem. Soc. 2018, 140, 3156–3169;
- 26cL.-J. Song, T. Wang, X. Zhang, L. W. Chung, Y.-D. Wu, ACS Catal. 2017, 7, 1361–1368;
- 26dC. Zhu, X. Zhou, H. Xing, K. An, J. Zhu, H. Xia, Angew. Chem. Int. Ed. 2015, 54, 3102–3106; Angew. Chem. 2015, 127, 3145–3149.
- 27T. Luo, R. Zhang, W. Zhang, X. Shen, T. Umemoto, J. Hu, Org. Lett. 2014, 16, 888–891.
- 28
- 28aG. Meißner, D. Dirican, C. Jäger, T. Braun, E. Kemnitz, Catal. Sci. Technol. 2017, 7, 3348–3354;
- 28bB. Calvo, C. P. Marshall, T. Krahl, J. Kröhnert, A. Trunschke, G. Scholz, T. Braun, E. Kemnitz, Dalton Trans. 2018, 47, 16461–16473.
- 29The counteranion of 5 could not be identified until [BF4]− was observed after a few hours. Note that HF can react with Bpin derivatives to form BF3 and then, [BF4]−. In fact, when Et3N/CsCO3 is added to trap HF, full conversion to complex 4 is observed.
- 30S. Scheiner, J. Phys. Chem. A 2018, 122, 2550–2562.
- 31
- 31aP. Tian, C. Feng, T.-P. Loh, Nat. Commun. 2015, 6, 7472;
- 31bD. Yu, L. Lu, Q. Shen, Org. Lett. 2013, 15, 940–943;
- 31cD. Zell, U. Dhawa, V. Müller, M. Bursch, S. Grimme, L. Ackermann, ACS Catal. 2017, 7, 4209–4213.