Nickel-Catalyzed Asymmetric Hydrogenation of N-Sulfonyl Imines
Bowen Li
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 P. R. China
Search for more papers by this authorDr. Jianzhong Chen
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 P. R. China
Search for more papers by this authorDr. Zhenfeng Zhang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 P. R. China
Search for more papers by this authorProf. Ilya D. Gridnev
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai, 9808578 Japan
Search for more papers by this authorCorresponding Author
Prof. Wanbin Zhang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 P. R. China
Search for more papers by this authorBowen Li
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 P. R. China
Search for more papers by this authorDr. Jianzhong Chen
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 P. R. China
Search for more papers by this authorDr. Zhenfeng Zhang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 P. R. China
Search for more papers by this authorProf. Ilya D. Gridnev
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai, 9808578 Japan
Search for more papers by this authorCorresponding Author
Prof. Wanbin Zhang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 P. R. China
Search for more papers by this authorGraphical Abstract
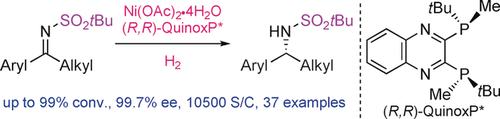
The first Ni-catalyzed asymmetric hydrogenation of N-sulfonyl imines using H2 gas as a hydrogen source has been realized with excellent enantioselectivity. The presence of an excess of the nickel salt, with respect to the ligand, promotes the formation of the active Ni catalyst. The reaction features a wide substrate scope, low catalyst loading, and convenient removal of the protecting group.
Abstract
An efficient nickel-catalyzed asymmetric hydrogenation of N-tBu-sulfonyl imines was developed with excellent yields and enantioselectivities using (R,R)-QuinoxP* as a chiral ligand. The use of a much lower catalyst loading (0.0095 mol %, S/C=10500) represents the highest catalytic activity for the Ni-catalyzed asymmetric hydrogenations reported so far. Mechanistic studies suggest that a coordination equilibrium exists between the nickel salt and its complex, and that excess nickel salt promotes the formation of the active Ni-complex, and therefore improved the efficiency of the hydrogenation. The catalytic cycle was also investigated by calculations to determine the origin of the enantioselectivity. An extensive network of numerous weak attractive interactions was found to exist between the catalyst and substrate in the transition state and may also contribute to the high catalytic activity.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201902576-sup-0001-misc_information.pdf9.9 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Chiral Amine Synthesis: Methods, Developments and Applications (Ed.: ), Wiley-VCH, Weinheim, 2010.
- 2For representative reviews, see:
- 2aH.-U. Blaser, C. Malan, B. Pugin, F. Spindler, H. Steiner, M. Studer, Adv. Synth. Catal. 2003, 345, 103;
- 2bW. Tang, X. Zhang, Chem. Rev. 2003, 103, 3029;
- 2cW. Zhang, Y. Chi, X. Zhang, Acc. Chem. Res. 2007, 40, 1278;
- 2dN. B. Johnson, I. C. Lennon, P. H. Moran, J. A. Ramsden, Acc. Chem. Res. 2007, 40, 1291;
- 2eN. Fleury-Brégeot, V. de la Fuente, S. Castillón, C. Claver, ChemCatChem 2010, 2, 1346;
- 2fC. Wang, B. Villa-Marcos, J. Xiao, Chem. Commun. 2011, 47, 9773;
- 2gJ.-H. Xie, S.-F. Zhu, Q.-L. Zhou, Chem. Rev. 2011, 111, 1713;
- 2hD. J. Ager, A. H. M. de Vries, J. G. de Vries, Chem. Soc. Rev. 2012, 41, 3340;
- 2iZ. Yu, W. Jin, Q. Jiang, Angew. Chem. Int. Ed. 2012, 51, 6060; Angew. Chem. 2012, 124, 6164;
- 2jJ.-H. Xie, Q.-L. Zhou, Acta Chim. Sinica 2012, 70, 1427;
- 2kQ.-A. Chen, Z.-S. Ye, Y. Duan, Y.-G. Zhou, Chem. Soc. Rev. 2013, 42, 497;
- 2lP. Etayo, A. Vidal-Ferran, Chem. Soc. Rev. 2013, 42, 728;
- 2mK. H. Hopmann, A. Bayer, Coord. Chem. Rev. 2014, 268, 59;
- 2nZ. Zhang, N. A. Butt, W. Zhang, Chem. Rev. 2016, 116, 14769;
- 2oP.-G. Echeverria, T. Ayad, P. Phansavath, V. Ratovelomanana-Vidal, Synthesis 2016, 48, 2523;
- 2pC. S. G. Seo, R. H. Morris, Organometallics 2019, 38, 47.
- 3For reviews, see:
- 3aR. H. Morris, Chem. Soc. Rev. 2009, 38, 2282;
- 3bK. Gopalaiah, Chem. Rev. 2013, 113, 3248;
- 3cH. Pellissier, H. Clavier, Chem. Rev. 2014, 114, 2775;
- 3dR. H. Morris, Acc. Chem. Res. 2015, 48, 1494;
- 3eY.-Y. Li, S.-L. Yu, W.-Y. Shen, J.-X. Gao, Acc. Chem. Res. 2015, 48, 2587;
- 3fP. J. Chirik, Acc. Chem. Res. 2015, 48, 1687;
- 3gI. Bauer, H.-J. Knölker, Chem. Rev. 2015, 115, 3170;
- 3hR. Bigler, A. Mezzetti, Org. Process Res. Dev. 2016, 20, 253;
- 3iZ. Zhang, N. A. Butt, M. Zhou, D. Liu, W. Zhang, Chin. J. Chem. 2018, 36, 443.
- 4
- 4aP. O. Lagaditis, P. E. Sues, J. F. Sonnenberg, K. Y. Wan, A. J. Lough, R. H. Morris, J. Am. Chem. Soc. 2014, 136, 1367;
- 4bM. R. Friedfeld, H. Zhong, R. T. Ruck, M. Shevlin, P. J. Chirik, Science 2018, 360, 888;
- 4cL. Zhang, Y. Tang, Z. Han, K. Ding, Angew. Chem. Int. Ed. 2019, 58, 4973; Angew. Chem. 2019, 131, 5027.
- 5
- 5aY. Hamada, Y. Koseki, T. Fujii, T. Maeda, T. Hibino, K. Makino, Chem. Commun. 2008, 46, 6206;
- 5bT. Hibino, K. Makino, T. Sugiyama, Y. Hamada, ChemCatChem 2009, 1, 237.
- 6M. Shevlin, M. R. Friedfeld, H. Sheng, N. A. Pierson, J. M. Hoyt, L.-C. Campeau, P. J. Chirik, J. Am. Chem. Soc. 2016, 138, 3562.
- 7
- 7aW. Gao, H. Lv, T. Zhang, Y. Yang, L. W. Chung, Y.-D. Wu, X. Zhang, Chem. Sci. 2017, 8, 6419;
- 7bX. Li, C. You, S. Li, H. Lv, X. Zhang, Org. Lett. 2017, 19, 5130;
- 7cJ. Long, W. Gao, Y. Guan, H. Lv, X. Zhang, Org. Lett. 2018, 20, 5914;
- 7dY.-Q. Guan, Z. Han, X. Li, C. You, X. Tan, H. Lv, X. Zhang, Chem. Sci. 2019, 10, 252.
- 8
- 8aH. Xu, P. Yang, P. Chuanprasit, H. Hirao, J. Zhou, Angew. Chem. Int. Ed. 2015, 54, 5112; Angew. Chem. 2015, 127, 5201;
- 8bP. Yang, L. H. Lim, P. Chuanprasit, H. Hirao, J. Zhou, Angew. Chem. Int. Ed. 2016, 55, 12083; Angew. Chem. 2016, 128, 12262;
- 8cX. Zhao, H. Xu, X. Huang, J. S. Zhou, Angew. Chem. Int. Ed. 2019, 58, 292; Angew. Chem. 2019, 131, 298.
- 9For representative papers, see:
- 9aF. Tian, D. Yao, Y. Liu, F. Xie, W. Zhang, Adv. Synth. Catal. 2010, 352, 1841;
- 9bY. Liu, W. Zhang, Angew. Chem. Int. Ed. 2013, 52, 2203; Angew. Chem. 2013, 125, 2259;
- 9cJ. Chen, D. Liu, N. Butt, C. Li, D. Fan, Y. Liu, W. Zhang, Angew. Chem. Int. Ed. 2013, 52, 11632; Angew. Chem. 2013, 125, 11846;
- 9dY. Liu, I. D. Gridnev, W. Zhang, Angew. Chem. Int. Ed. 2014, 53, 1901; Angew. Chem. 2014, 126, 1932;
- 9eQ. Hu, Z. Zhang, Y. Liu, T. Imamoto, W. Zhang, Angew. Chem. Int. Ed. 2015, 54, 2260; Angew. Chem. 2015, 127, 2288;
- 9fJ. Chen, Z. Zhang, D. Liu, W. Zhang, Angew. Chem. Int. Ed. 2016, 55, 8444; Angew. Chem. 2016, 128, 8584;
- 9gJ. Chen, Z. Zhang, B. Li, F. Li, Y. Wang, M. Zhao, I. D. Gridnev, T. Imamoto, W. Zhang, Nat. Commun. 2018, 9, 5000.
- 10Y.-C. Chen, T.-F. Wu, J.-G. Deng, H. Liu, X. Cui, J. Zhu, Y.-Z. Jiang, M. C. K. Choi, A. S. C. Chan, J. Org. Chem. 2002, 67, 5301.
- 11D. Enders, M. Seppelt, T. Beck, Adv. Synth. Catal. 2010, 352, 1413.