Synthesis of Tri- and Difluoromethoxylated Compounds by Visible-Light Photoredox Catalysis
Johnny W. Lee
Department of Chemistry and Institute of Chemical Biology and Drug Discovery, Stony Brook University, Stony Brook, NY, 11794 USA
Search for more papers by this authorDr. Katarzyna N. Lee
Department of Chemistry and Institute of Chemical Biology and Drug Discovery, Stony Brook University, Stony Brook, NY, 11794 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Ming-Yu Ngai
Department of Chemistry and Institute of Chemical Biology and Drug Discovery, Stony Brook University, Stony Brook, NY, 11794 USA
Search for more papers by this authorJohnny W. Lee
Department of Chemistry and Institute of Chemical Biology and Drug Discovery, Stony Brook University, Stony Brook, NY, 11794 USA
Search for more papers by this authorDr. Katarzyna N. Lee
Department of Chemistry and Institute of Chemical Biology and Drug Discovery, Stony Brook University, Stony Brook, NY, 11794 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Ming-Yu Ngai
Department of Chemistry and Institute of Chemical Biology and Drug Discovery, Stony Brook University, Stony Brook, NY, 11794 USA
Search for more papers by this authorDedicated to Professor Amir Hoveyda on the occasion of his 60th birthday
Graphical Abstract
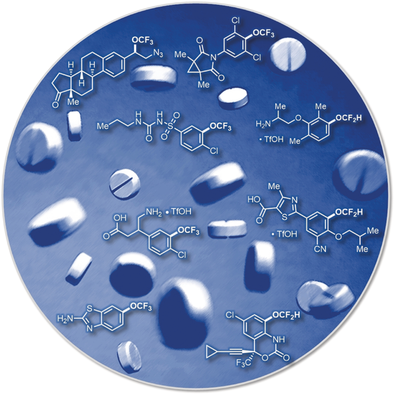
Illuminating OCF3 and OCF2H: Tri- and difluoromethyl ethers are privileged moieties in the realm of medicinal chemistry and are found in marketed pharmaceuticals and agrichemicals. The recent advances in visible-light photocatalytic synthesis of these moieties will likely render their introduction routine as a part of drug design and drug discovery processes.
Abstract
Trifluoromethoxy (OCF3) and difluoromethoxy (OCF2H) groups are fluorinated structural motifs that exhibit unique physicochemical characteristics. Incorporation of these substituents into organic molecules is a highly desirable approach used in medicinal chemistry and drug discovery processes to alter the properties of a parent compound. Recently, tri- and difluoromethyl ethers have received increasing attention and several innovative strategies to access these valuable functional groups have been developed. The focus of this Minireview is the use of visible-light photoredox catalysis in the synthesis of tri- and difluoromethyl ethers. Recent photocatalytic strategies for the formation of O−CF3, C−OCF3, O−CF2H, and C−OCF2H bonds as well as other transformations leading to the construction of ORF groups are discussed herein.
Conflict of interest
The authors declare no conflict of interest.
References
- 1
- 1aS. Purser, P. R. Moore, S. Swallow, V. Gouverneur, Chem. Soc. Rev. 2008, 37, 320;
- 1bI. Ojima, Fluorine in Medicinal Chemistry and Chemical Biology, Blackwell Publishing Ltd, Chichester, 2009;
10.1002/9781444312096 Google Scholar
- 1cT. Liang, C. N. Neumann, T. Ritter, Angew. Chem. Int. Ed. 2013, 52, 8214; Angew. Chem. 2013, 125, 8372;
- 1dJ. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok, H. Liu, Chem. Rev. 2014, 114, 2432;
- 1eY. Zhou, J. Wang, Z. Gu, S. Wang, W. Zhu, J. L. Aceña, V. A. Soloshonok, K. Izawa, H. Liu, Chem. Rev. 2016, 116, 422.
- 2
- 2aF. Leroux, P. Jeschke, M. Schlosser, Chem. Rev. 2005, 105, 827;
- 2bK. Muller, C. Faeh, F. Diederich, Science 2007, 317, 1881;
- 2cP. Jeschke, E. Baston, F. R. Leroux, Mini-Rev. Med. Chem. 2007, 7, 1027;
- 2dG. Landelle, A. Panossian, F. R. Leroux, Curr. Top. Med. Chem. 2014, 14, 941.
- 3L. Xing, D. C. Blakemore, A. Narayanan, R. Unwalla, F. Lovering, R. A. Denny, H. Zhou, M. E. Bunnage, ChemMedChem 2015, 10, 715.
- 4M. A. Mcclinton, D. A. Mcclinton, Tetrahedron 1992, 48, 6555.
- 5C. Hansch, A. Leo, Substituent Constants for Correlation Analysis in Chemistry and Biology, Wiley, New York, 1979.
- 6D. Federsel, A. Herrmann, D. Christen, S. Sander, H. Willner, H. Oberhammer, J. Mol. Struct. 2001, 567, 127.
- 7
- 7aK. Müller, Chimia 2014, 68, 356;
- 7bQ. A. Huchet, N. Trapp, B. Kuhn, B. Wagner, H. Fischer, N. A. Kratochwil, E. M. Carreira, K. Müller, J. Fluorine Chem. 2017, 198, 34.
- 8
- 8aM. J. Waring, Expert Opin. Drug Discovery 2010, 5, 235;
- 8bA. Gaulton, L. J. Bellis, A. P. Bento, J. Chambers, M. Davies, A. Hersey, Y. Light, S. McGlinchey, D. Michalovich, B. Al-Lazikani, J. P. Overington, Nucleic Acids Res. 2012, 40, D 1100.
- 9C. B. Burness, Drugs 2015, 75, 1559.
- 10A. Milane, S. Vautier, H. Chacun, V. Meininger, G. Bensimon, R. Farinotti, C. Fernandez, Neurosci. Lett. 2009, 452, 12.
- 11L. M. Dias, Clin. Drug Invest. 2009, 29, 3.
- 12J. Chong, B. Leung, P. Poole, Cochrane Database Syst. Rev. 2017, https://doi.org/10.1002/14651858.CD002309.pub5.
- 13Selected recent reviews on trifluoromethoxylation reactions:
- 13aK. N. Lee, J. W. Lee, M.-Y. Ngai, Synlett 2016, 27, 313;
- 13bT. Besset, P. Jubault, X. Pannecoucke, T. Poisson, Org. Chem. Front. 2016, 3, 1004;
- 13cA. Tlili, F. Toulgoat, T. Billard, Angew. Chem. Int. Ed. 2016, 55, 11726; Angew. Chem. 2016, 128, 11900;
- 13dK. N. Lee, J. W. Lee, M.-Y. Ngai, Tetrahedron 2018, 74, 7127;
- 13eX. T. Zhang, P. Tang, Sci. China Chem. 2019, 62, 525.
- 14C. Ni, J. Hu, Synthesis 2014, 46, 842.
- 15Selected reviews on photoredox catalysis:
- 15aC. K. Prier, D. A. Rankic, D. W. C. MacMillan, Chem. Rev. 2013, 113, 5322;
- 15bM. D. Kärkäs, J. A. Porco, Jr., C. R. Stephenson, Chem. Rev. 2016, 116, 9683;
- 15cN. A. Romero, D. A. Nicewicz, Chem. Rev. 2016, 116, 10075;
- 15dK. L. Skubi, T. R. Blum, T. P. Yoon, Chem. Rev. 2016, 116, 10035;
- 15eJ. K. Matsui, S. B. Lang, D. R. Heitz, G. A. Molander, ACS Catal. 2017, 7, 2563.
- 16D. M. Schultz, T. P. Yoon, Science 2014, 343, 1239176.
- 17B. Sahoo, M. N. Hopkinson, Angew. Chem. Int. Ed. 2018, 57, 7942; Angew. Chem. 2018, 130, 8070.
- 18
- 18aM. E. Redwood, C. J. Willis, Can. J. Chem. 1965, 43, 1893;
- 18bW. B. Farnham, B. E. Smart, W. J. Middleton, J. C. Calabrese, D. A. Dixon, J. Am. Chem. Soc. 1985, 107, 4565;
- 18cM. Nishida, A. Vij, R. L. Kirchmeier, J. M. Shreeve, Inorg. Chem. 1995, 34, 6085;
- 18dK. O. Christe, J. Hegge, B. Hoge, R. Haiges, Angew. Chem. Int. Ed. 2007, 46, 6155; Angew. Chem. 2007, 119, 6267.
- 19
- 19aJ. W. Lee, D. N. Spiegowski, M.-Y. Ngai, Chem. Sci. 2017, 8, 6066;
- 19bT. M. Swager, K. Yoshinaga, Synfacts 2017, 13, 1142.
10.1055/s-0036-1591412 Google Scholar
- 20
- 20aK. N. Hojczyk, P. J. Feng, C. B. Zhan, M. Y. Ngai, Angew. Chem. Int. Ed. 2014, 53, 14559; Angew. Chem. 2014, 126, 14787;
- 20bP. J. Feng, K. N. Lee, J. W. Lee, C. B. Zhan, M. Y. Ngai, Chem. Sci. 2016, 7, 424;
- 20cK. N. Lee, Z. Lei, C. A. Morales-Rivera, P. Liu, M. Y. Ngai, Org. Biomol. Chem. 2016, 14, 5599.
- 21J. W. Beatty, J. J. Douglas, K. P. Cole, C. R. J. Stephenson, Nat. Commun. 2015, 6, 7919.
- 22A. Juris, V. Balzani, P. Belser, A. von Zelewsky, Helv. Chim. Acta 1981, 64, 2175.
- 23
- 23aA. Studer, Angew. Chem. Int. Ed. 2012, 51, 8950; Angew. Chem. 2012, 124, 9082;
- 23bM. Nappi, G. Bergonzini, P. Melchiorre, Angew. Chem. Int. Ed. 2014, 53, 4921; Angew. Chem. 2014, 126, 5021.
- 24
- 24aA. Studer, Chem. Eur. J. 2001, 7, 1159;
10.1002/1521-3765(20010316)7:6<1159::AID-CHEM1159>3.0.CO;2-I CAS PubMed Web of Science® Google Scholar
- 24bH. Fischer, Chem. Rev. 2001, 101, 3581.
- 25F. Cong, Y. Wei, P. Tang, Chem. Commun. 2018, 54, 4473.
- 26D. H. R. Barton, L. S. Godinho, R. H. Hesse, M. M. Pechet, Chem. Commun. 1968, 804.
- 27
- 27aW. J. Peláez, G. A. Argüello, Tetrahedron Lett. 2010, 51, 5242;
- 27bF. Venturini, W. Navarrini, A. Famulari, M. Sansotera, P. Dardani, V. Tortelli, J. Fluorine Chem. 2012, 140, 43.
- 28W. Zheng, C. A. Morales-Rivera, J. W. Lee, P. Liu, M. Y. Ngai, Angew. Chem. Int. Ed. 2018, 57, 9645; Angew. Chem. 2018, 130, 9793.
- 29
- 29aD. A. Nagib, D. W. MacMillan, Nature 2011, 480, 224;
- 29bT. Cernak, K. D. Dykstra, S. Tyagarajan, P. Vachal, S. W. Krska, Chem. Soc. Rev. 2016, 45, 546.
- 30W. Zheng, J. W. Lee, C. A. Morales-Rivera, P. Liu, M.-Y. Ngai, Angew. Chem. Int. Ed. 2018, 57, 13795; Angew. Chem. 2018, 130, 13991.
- 31OCF3-pyridinium salt was first synthesized by Umemoto and Hu for trifluoromethoxylation of electron-rich arenes in the absence of photoredox catalyst. See: T. Umemoto, J. Hu, CN105017143A, China, 2015.
- 32For application of OCF3-pyridinium salts for radical trifluoromethoxylation of (hetero)arenes under photoredox-catalyzed conditions, see: B. J. Jelier, P. F. Tripet, E. Pietrasiak, I. Franzoni, G. Jeschke, A. Togni, Angew. Chem. Int. Ed. 2018, 57, 13784; Angew. Chem. 2018, 130, 13980.
- 33Synthesis of aryl difluoromethyl ethers from phenols. For selected examples of stoichiometric reactions, see:
- 33aL. Zhang, J. Zheng, J. Hu, J. Org. Chem. 2006, 71, 9845;
- 33bJ. Zheng, Y. Li, L. Zhang, J. Hu, G. J. Meuzelaar, H.-J. Federsel, Chem. Commun. 2007, 5149;
- 33cY. Zafrani, G. Sod-Moriah, Y. Segall, Tetrahedron 2009, 65, 5278;
- 33dF. Wang, W. Huang, J. Hu, Chin. J. Chem. 2011, 29, 2717;
- 33eF. Wang, L. Zhang, J. Zheng, J. Hu, J. Fluorine Chem. 2011, 132, 521;
- 33fJ. B. Sperry, K. Sutherland, Org. Process Res. Dev. 2011, 15, 721;
- 33gP. S. Fier, J. F. Hartwig, Angew. Chem. Int. Ed. 2013, 52, 2092; Angew. Chem. 2013, 125, 2146;
- 33hC. S. Thomoson, W. R. Dolbier, Jr., J. Org. Chem. 2013, 78, 8904;
- 33iL. Li, F. Wang, C. Ni, J. Hu, Angew. Chem. Int. Ed. 2013, 52, 12390; Angew. Chem. 2013, 125, 12616; for examples of other indirect, multistep strategies, see:
- 33jY. Hagooly, O. Cohen, S. Rozen, Tetrahedron Lett. 2009, 50, 392;
- 33kW. R. Dolbier, Jr., F. Wang, X. Tang, C. S. Thomoson, L. Wang, J. Fluorine Chem. 2014, 160, 72; for examples of catalytic O−CF2H bond-forming reactions, see:
- 33lK. Levchenko, O. P. Datsenko, O. Serhiichuk, A. Tolmachev, V. O. Iaroshenko, P. K. Mykhailiuk, J. Org. Chem. 2016, 81, 5803;
- 33mJ. Yang, M. Jiang, Y. Jin, H. Yang, H. Fu, Org. Lett. 2017, 19, 2758.
- 34J. M. Birchall, G. E. Cross, R. N. Haszeldine, Proc. Chem. Soc. London 1960, 81.
- 35F. Wang, T. Luo, J. Hu, Y. Wang, H. S. Krishnan, P. V. Jog, S. K. Ganesh, G. K. S. Prakash, G. A. Olah, Angew. Chem. Int. Ed. 2011, 50, 7153; Angew. Chem. 2011, 123, 7291.
- 36
- 36aJ. W. Lee, W. Zheng, C. A. Morales-Rivera, P. Liu, M. Y. Ngai, Chem. Sci. 2019, 10, 3217;
- 36bP. Knochel, M. Balkenhohl, Synfacts 2019, 15, 520.
- 37L. Flamigni, A. Barbieri, C. Sabatini, B. Ventura, F. Barigelletti, Top. Curr. Chem. 2007, 281, 143.
- 38After this Minireview was accepted for publication, a photocatalytic, copper-mediated trifluoromethoxylation of arenediazonium tetrafluoroborates was reported; see: S. Yang, M. Chen, P. Tang, Angew. Chem. Int. Ed. 2019, https://doi.org/10.1002/anie.201901447; Angew. Chem. 2019, DOI: https://doi.org/10.1002/ange.201901447.