Paired Electrochemical Reactions and the On-Site Generation of a Chemical Reagent
Graphical Abstract
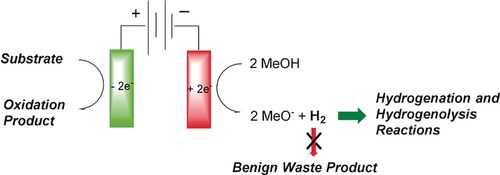
An alcohol oxidation, an oxidative condensation, intramolecular anodic olefin coupling reactions, an amide oxidation, and a mediated oxidation were all shown to be compatible with the generation and use of hydrogen gas at the cathode. This pairing of an electrolysis reaction with the production of a chemical reagent or substrate has the potential to greatly expand the use of more energy efficient paired electrochemical reactions.
Abstract
While the majority of reported paired electrochemical reactions involve carefully matched cathodic and anodic reactions, the precise matching of half reactions in an electrolysis cell is not generally necessary. During a constant current electrolysis almost any oxidation and reduction reaction can be paired, and in the presented work we capitalize on this observation by examining the coupling of anodic oxidation reactions with the production of hydrogen gas for use as a reagent in remote, Pd-catalyzed hydrogenation and hydrogenolysis reactions. To this end, an alcohol oxidation, an oxidative condensation, intramolecular anodic olefin coupling reactions, an amide oxidation, and a mediated oxidation were all shown to be compatible with the generation and use of hydrogen gas at the cathode. This pairing of an electrolysis reaction with the production of a chemical reagent or substrate has the potential to greatly expand the use of more energy efficient paired electrochemical reactions.