Asymmetric Alkenylation of Enones and Imines Enabled by A Highly Efficient Aryl to Vinyl 1,4-Rhodium Migration
Dr. Shu-Sheng Zhang
Innovation Research Institute of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Tian-Jiao Hu
Key Laboratory of Synthetic Chemistry of Natural Substances, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200032 China
These authors contributed equally to this work.
Search for more papers by this authorMeng-Yao Li
Key Laboratory of Synthetic Chemistry of Natural Substances, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200032 China
Search for more papers by this authorYi-Kang Song
Innovation Research Institute of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203 China
Search for more papers by this authorProf. Xiao-Di Yang
Innovation Research Institute of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203 China
Search for more papers by this authorCorresponding Author
Prof. Chen-Guo Feng
Innovation Research Institute of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203 China
Key Laboratory of Synthetic Chemistry of Natural Substances, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Prof. Guo-Qiang Lin
Innovation Research Institute of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203 China
Key Laboratory of Synthetic Chemistry of Natural Substances, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200032 China
Search for more papers by this authorDr. Shu-Sheng Zhang
Innovation Research Institute of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Tian-Jiao Hu
Key Laboratory of Synthetic Chemistry of Natural Substances, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200032 China
These authors contributed equally to this work.
Search for more papers by this authorMeng-Yao Li
Key Laboratory of Synthetic Chemistry of Natural Substances, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200032 China
Search for more papers by this authorYi-Kang Song
Innovation Research Institute of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203 China
Search for more papers by this authorProf. Xiao-Di Yang
Innovation Research Institute of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203 China
Search for more papers by this authorCorresponding Author
Prof. Chen-Guo Feng
Innovation Research Institute of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203 China
Key Laboratory of Synthetic Chemistry of Natural Substances, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Prof. Guo-Qiang Lin
Innovation Research Institute of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203 China
Key Laboratory of Synthetic Chemistry of Natural Substances, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200032 China
Search for more papers by this authorGraphical Abstract
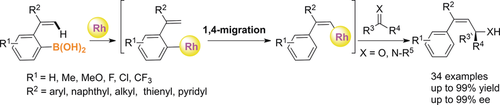
The asymmetric rhodium-catalyzed alkenylation of enones and imines with arylboronic acids has been developed. A highly controllable aryl to vinyl 1,4-rhodium migration is the key step. Stereodefined vinyl moieties were installed in excellent enantioselectivies for most examined examples. DFT calculations reveal that the driving force of this rhodium migration is a kinetically favored process.
Abstract
The asymmetric rhodium-catalyzed alkenylation of enones and imines with arylboronic acids has been developed. A highly controllable aryl to vinyl 1,4-rhodium migration is the key step. Stereodefined vinyl moieties were installed in excellent enantioselectivies for most examined examples. DFT calculations reveal that the driving force of this rhodium migration is a kinetically favored process.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201813585-sup-0001-misc_information.pdf21.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see:
- 1aK. Fagnou, M. Lautens, Chem. Rev. 2003, 103, 169;
- 1bT. Hayashi, K. Yamasaki, Chem. Rev. 2003, 103, 2829;
- 1cH. J. Edwards, J. D. Hargrave, S. D. Penrose, C. G. Frost, Chem. Soc. Rev. 2010, 39, 2093;
- 1dC. S. Marques, A. J. Burke, ChemCatChem 2011, 3, 635;
- 1eP. Tian, H.-Q. Dong, G.-Q. Lin, ACS Catal. 2012, 2, 95;
- 1fM. Jean, B. Casanova, S. Gnoatto, P. van de Weghe, Org. Biomol. Chem. 2015, 13, 9168;
- 1gM. M. Heravi, M. Dehghani, V. Zadsirjan, Tetrahedron: Asymmetry 2016, 27, 513.
- 2For selected examples, see: Addition to C=C bonds:
- 2aA. Duursma, J.-G. Boiteau, L. Lefort, J. A. F. Boogers, A. H. M. de Vries, J. G. de Vries, A. J. Minnaard, B. L. Feringa, J. Org. Chem. 2004, 69, 8045;
- 2bG. Lalic, E. J. Corey, Tetrahedron Lett. 2008, 49, 4894;
- 2cH.-J. Yu, C. Shao, Z. Cui, C.-G. Feng, G.-Q. Lin, Chem. Eur. J. 2012, 18, 13274; Addition to C=N bonds:
- 2dY. Luo, A. J. Carnell, H. W. Lam, Angew. Chem. Int. Ed. 2012, 51, 6762; Angew. Chem. 2012, 124, 6866;
- 2eZ. Cui, Y.-J. Chen, W.-Y. Gao, C.-G. Feng, G.-Q. Lin, Org. Lett. 2014, 16, 1016;
- 2fY. Wang, Y. Liu, D. Zhang, H. Wei, M. Shi, F. Wang, Angew. Chem. Int. Ed. 2016, 55, 3776; Angew. Chem. 2016, 128, 3840;
- 2gX.-W. Qian, Z.-J. Xue, Q. Zhao, Z. Cui, Y.-J. Chen, C.-G. Feng, G.-Q. Lin, Org. Lett. 2017, 19, 5601.
- 3
- 3aM. Daini, A. Yamamoto, M. Suginome, J. Am. Chem. Soc. 2008, 130, 2918;
- 3bC. Wang, Z. Xu, T. Tobrman, E. I. Negishi, Adv. Synth. Catal. 2010, 352, 627;
- 3cR. Alfaro, A. Parra, J. Alemán, J. L. G. Ruano, M. Tortosa, J. Am. Chem. Soc. 2012, 134, 15165;
- 3dH. Yoshida, I. Kageyuki, K. Takaki, Org. Lett. 2013, 15, 952;
- 3eT.-J. Hu, G. Zhang, Y.-H. Chen, C.-G. Feng, G.-Q. Lin, J. Am. Chem. Soc. 2016, 138, 2897.
- 4K. Sasaki, T. Nishimura, R. Shintani, E. A. B. Kantchev, T. Hayashi, Chem. Sci. 2012, 3, 1278.
- 5H. B. Hepburn, H. W. Lam, Angew. Chem. Int. Ed. 2014, 53, 11605; Angew. Chem. 2014, 126, 11789.
- 6K. Oguma, M. Miura, T. Satoh, M. Nomura, J. Am. Chem. Soc. 2000, 122, 10464.
- 7For reviews, see:
- 7aS. Ma, Z. Gu, Angew. Chem. Int. Ed. 2005, 44, 7512; Angew. Chem. 2005, 117, 7680;
- 7bF. Shi, R. C. Larock, Top. Curr. Chem. 2010, 292, 123.
- 8For selected examples on vinyl to aryl 1,4-rhodium(I) migration, see:
- 8aT. Hayashi, K. Inoue, N. Taniguchi, M. Ogasawara, J. Am. Chem. Soc. 2001, 123, 9918;
- 8bT. Miura, T. Sasaki, H. Nakazawa, M. Murakami, J. Am. Chem. Soc. 2005, 127, 1390;
- 8cR. Shintani, K. Okamoto, T. Hayashi, J. Am. Chem. Soc. 2005, 127, 2872;
- 8dH. Yamabe, A. Mizuno, H. Kusama, N. Iwasawa, J. Am. Chem. Soc. 2005, 127, 3248;
- 8eR. Shintani, K. Yashio, T. Nakamura, K. Okamoto, T. Shimada, T. Hayashi, J. Am. Chem. Soc. 2006, 128, 2772;
- 8fR. Shintani, S. Isobe, M. Takeda, T. Hayashi, Angew. Chem. Int. Ed. 2010, 49, 3795; Angew. Chem. 2010, 122, 3883;
- 8gM. Onoe, K. Baba, Y. Kim, Y. Kita, M. Tobisu, N. Chatani, J. Am. Chem. Soc. 2012, 134, 19477.
- 9For selected examples on alkyl to aryl 1,4-rhodium(I) migration, see:
- 9aT. Matsuda, M. Shigeno, M. Murakami, J. Am. Chem. Soc. 2007, 129, 12086;
- 9bF. Menard, M. Lautens, Angew. Chem. Int. Ed. 2008, 47, 2085; Angew. Chem. 2008, 120, 2115;
- 9cT. Seiser, O. A. Roth, N. Cramer, Angew. Chem. Int. Ed. 2009, 48, 6320; Angew. Chem. 2009, 121, 6438;
- 9dT. Seiser, N. Cramer, Angew. Chem. Int. Ed. 2010, 49, 10163; Angew. Chem. 2010, 122, 10361;
- 9eR. Shintani, R. Iino, K. Nozaki, J. Am. Chem. Soc. 2014, 136, 7849;
- 9fT. Sawano, M. Hashizume, S. Nishimoto, K. Ou, T. Nishimura, Org. Lett. 2015, 17, 2630.
- 10For examples on vinyl to allyl 1,4-rhodium(I) migration, see:
- 10aM. Callingham, B. M. Partridge, W. Lewis, H. W. Lam, Angew. Chem. Int. Ed. 2017, 56, 16352; Angew. Chem. 2017, 129, 16570;
- 10bB. M. Partridge, M. Callingham, W. Lewis, H. W. Lam, Angew. Chem. Int. Ed. 2017, 56, 7227; Angew. Chem. 2017, 129, 7333.
- 11For examples on 1,3- or 1,5-rhodium(I) migration, see:
- 11aJ. Zhang, J.-F. Liu, A. Ugrinov, A. F. X. Pillai, Z.-M. Sun, P. Zhao, J. Am. Chem. Soc. 2013, 135, 17270;
- 11bA. Masarwa, M. Weber, R. Sarpong, J. Am. Chem. Soc. 2015, 137, 6327;
- 11cM. Tobisu, J. Hasegawa, Y. Kita, H. Kinuta, N. Chatani, Chem. Commun. 2012, 48, 11437;
- 11dN. Ishida, Y. Shimamoto, T. Yano, M. Murakami, J. Am. Chem. Soc. 2013, 135, 19103.
- 12For selected examples on 1,4-rhodium(III) migration, see:
- 12aD. J. Burns, H. W. Lam, Angew. Chem. Int. Ed. 2014, 53, 9931; Angew. Chem. 2014, 126, 10089;
- 12bS. Guo, K. Yuan, M. Gu, A. Lin, H. Yao, Org. Lett. 2016, 18, 5236;
- 12cS. E. Korkis, D. J. Burns, H. W. Lam, J. Am. Chem. Soc. 2016, 138, 12252.
- 13For aryl to vinyl 1,4-rhodium migration in complex research, see: Y. Ikeda, K. Takano, M. Waragai, S. J. Kodama, N. Tsuchida, K. Takano, Y. Ishii, Organometallics 2014, 33, 2142.
- 14T.-J. Hu, M.-Y. Li, Q. Zhao, C.-G. Feng, G.-Q. Lin, Angew. Chem. Int. Ed. 2018, 57, 5871; Angew. Chem. 2018, 130, 5973.
- 15
- 15aJ. A. Labinger, J. E. Bercaw, Nature 2002, 417, 507;
- 15bS. H. Wiedemann, J. C. Lewis, J. A. Ellman, R. G. Bergman, J. Am. Chem. Soc. 2006, 128, 2452;
- 15cD. A. Colby, R. G. Bergman, J. A. Ellman, J. Am. Chem. Soc. 2008, 130, 3645.
- 16
- 16aZ.-Q. Wang, C.-G. Feng, M.-H. Xu, G.-Q. Lin, J. Am. Chem. Soc. 2007, 129, 5336;
- 16bS. Helbig, S. Sauer, N. Cramer, S. Laschat, A. Baro, W. Frey, Adv. Synth. Catal. 2007, 349, 2331.
- 17C.-G. Feng, Z.-Q. Wang, C. Shao, M.-H. Xu, G.-Q. Lin, Org. Lett. 2008, 10, 4101.
- 18K. Okamoto, T. Hayashi, V. H. Rawal, Chem. Commun. 2009, 4815.
- 19 Boronic Acids: Preparation and Applications in Organic Synthesis Medicine, and Materials, 2nd ed. ), Wiley-VCH, Weinheim, 2011.
- 20CCDC 1876119 (7 c) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre
- 21Y. Takaya, M. Ogasawara, T. Hayashi, M. Sakai, N. Miyaura, J. Am. Chem. Soc. 1998, 120, 5579.
- 22See the Supporting Information for computational details.
- 23D. V. Partyka, Chem. Rev. 2011, 111, 1529.