Cobalt-Catalyzed Intermolecular [2+2] Cycloaddition between Alkynes and Allenes
Dr. Wei Ding
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore, 637371 Singapore
Search for more papers by this authorCorresponding Author
Prof. Naohiko Yoshikai
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore, 637371 Singapore
Search for more papers by this authorDr. Wei Ding
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore, 637371 Singapore
Search for more papers by this authorCorresponding Author
Prof. Naohiko Yoshikai
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore, 637371 Singapore
Search for more papers by this authorGraphical Abstract
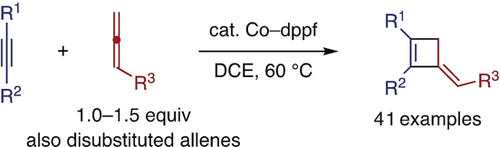
In equal measure: A cobalt/diphosphine catalyst promotes a selective [2+2] cycloaddition reaction between an alkyne and an allene, affording a 3-alkylidenecyclobutene with high regioselectivity. The reaction tolerates various internal alkynes, and mono- and disubstituted allenes with different functional groups, and can be achieved using a near equimolar mixture of the unsaturated reactants.
Abstract
An intermolecular [2+2] cycloaddition reaction between an alkyne and an allene is reported. In the presence of a cobalt(I)/diphosphine catalyst, a near equimolar mixture of the alkyne and allene is converted into a 3-alkylidenecyclobutene derivative in good yield with high regioselectivity. The reaction tolerates a variety of internal alkynes and mono- or disubstituted allenes bearing various functional groups. The reaction is proposed to involve regioselective oxidative cyclization of the alkyne and allene to form a 4-alkylidenecobaltacyclopentene intermediate, with subsequent C−C reductive elimination.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201813283-sup-0001-misc_information.pdf13.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aV. M. Dembitsky, J. Nat. Med. 2008, 62, 1–33;
- 1bV. M. Dembitsky, Phytomedicine 2014, 21, 1559–1581.
- 2
- 2aJ. C. Namyslo, D. E. Kaufmann, Chem. Rev. 2003, 103, 1485–1537;
- 2bT. Seiser, T. Saget, D. N. Tran, N. Cramer, Angew. Chem. Int. Ed. 2011, 50, 7740–7752; Angew. Chem. 2011, 123, 7884–7896;
- 2cG. Fumagalli, S. Stanton, J. F. Bower, Chem. Rev. 2017, 117, 9404–9432.
- 3
- 3aE. Lee-Ruff, G. Mladenova, Chem. Rev. 2003, 103, 1449–1483;
- 3bY. Xu, M. L. Conner, M. K. Brown, Angew. Chem. Int. Ed. 2015, 54, 11918–11928; Angew. Chem. 2015, 127, 12086–12097.
- 4B. Alcaide, P. Almendros, C. Aragoncillo, Chem. Soc. Rev. 2010, 39, 783–816.
- 5
- 5aK. M. Brummond, D. Chen, Org. Lett. 2005, 7, 3473–3475;
- 5bC. H. Oh, A. K. Gupta, D. I. Park, N. Kim, Chem. Commun. 2005, 5670–5672;
- 5cH. Ohno, T. Mizutani, Y. Kadoh, K. Miyamura, T. Tanaka, Angew. Chem. Int. Ed. 2005, 44, 5113–5115; Angew. Chem. 2005, 117, 5243–5245;
- 5dC. Mukai, Y. Hara, Y. Miyashita, F. Inagaki, J. Org. Chem. 2007, 72, 4454–4461;
- 5eH. Ohno, T. Mizutani, Y. Kadoh, A. Aso, K. Miyamura, N. Fujii, T. Tanaka, J. Org. Chem. 2007, 72, 4378–4389;
- 5fT. V. Ovaska, R. E. Kyne, Tetrahedron Lett. 2008, 49, 376–378;
- 5gO. Buisine, V. Gandon, L. Fensterbank, C. Aubert, M. Malacria, Synlett 2008, 751–754;
- 5hA. K. Mailyan, I. M. Krylov, C. Bruneau, P. H. Dixneuf, S. N. Osipov, Synlett 2011, 2321–2324.
- 6
- 6aQ. Shen, G. B. Hammond, J. Am. Chem. Soc. 2002, 124, 6534–6535;
- 6bC. H. Oh, D. I. Park, S. H. Jung, V. R. Reddy, A. K. Gupta, Y. M. Kim, Synlett 2005, 2092–2094;
- 6cN. Saito, Y. Tanaka, Y. Sato, Org. Lett. 2009, 11, 4124–4126.
- 7
- 7aB. E. Kirk, D. R. Taylor, J. Chem. Soc. Perkin Trans. 1 1974, 1844–1848;
- 7bD. J. Pasto, W. Kong, J. Org. Chem. 1988, 53, 4807–4810;
- 7cM. Kimura, Y. Horino, Y. Wakamiya, T. Okajima, Y. Tamaru, J. Am. Chem. Soc. 1997, 119, 10869–10870;
- 7dY. Horino, M. Kimura, S. Tanaka, T. Okajima, Y. Tamaru, Chem. Eur. J. 2003, 9, 2419–2438;
- 7eG. Maas, B. Manz, T. Mayer, U. Werz, Tetrahedron 1999, 55, 1309–1320.
- 8
- 8aK. Sakashita, Y. Shibata, K. Tanaka, Angew. Chem. Int. Ed. 2016, 55, 6753–6757; Angew. Chem. 2016, 128, 6865–6869;
- 8bM. Shanmugasundaram, M.-S. Wu, C.-H. Cheng, Org. Lett. 2001, 3, 4233–4236.
- 9
- 9aK. C. Chao, D. K. Rayabarapu, C.-C. Wang, C.-H. Cheng, J. Org. Chem. 2001, 66, 8804–8810;
- 9bJ. Treutwein, G. Hilt, Angew. Chem. Int. Ed. 2008, 47, 6811–6813; Angew. Chem. 2008, 120, 6916–6919;
- 9cG. Hilt, A. Paul, J. Treutwein, Org. Lett. 2010, 12, 1536–1539.
- 10A. Nishimura, E. Tamai, M. Ohashi, S. Ogoshi, Chem. Eur. J. 2014, 20, 6613–6617.
- 11V. V. Pagar, T. V. RajanBabu, Science 2018, 361, 68–72.
- 12For selected reviews on cobalt-catalyzed reductive coupling and cycloaddition, see:
- 12aW. Hess, J. Treutwein, G. Hilt, Synthesis 2008, 3537–3562;
- 12bM. Jeganmohan, C. H. Cheng, Chem. Eur. J. 2008, 14, 10876–10886;
- 12cP. Gandeepan, C.-H. Cheng, Acc. Chem. Res. 2015, 48, 1194–1206;
- 12dP. Röse, G. Hilt, Synthesis 2016, 48, 463–492.
- 13For examples of [2+2] cycloaddition between alkynes and alkenes using other transition metals, see:
- 13aT. Mitsudo, H. Naruse, T. Kondo, Y. Ozaki, Y. Watanabe, Angew. Chem. Int. Ed. Engl. 1994, 33, 580–581; Angew. Chem. 1994, 106, 595–597;
- 13bC. S. Yi, D. W. Lee, Y. Chen, Organometallics 1999, 18, 2043–2045;
- 13cD.-J. Huang, D. K. Rayabarapu, L.-P. Li, T. Sambaiah, C.-H. Cheng, Chem. Eur. J. 2000, 6, 3706–3713;
10.1002/1521-3765(20001016)6:20<3706::AID-CHEM3706>3.0.CO;2-P CAS PubMed Web of Science® Google Scholar
- 13dR. W. Jordan, W. Tam, Org. Lett. 2000, 2, 3031–3034;
- 13eT. Shibata, K. Takami, A. Kawachi, Org. Lett. 2006, 8, 1343–1345;
- 13fY. Kuninobu, P. Yu, K. Takai, Chem. Lett. 2007, 36, 1162–1163;
- 13gB.-M. Fan, X.-J. Li, F.-Z. Peng, H.-B. Zhang, A. S. C. Chan, Z.-H. Shao, Org. Lett. 2010, 12, 304–306;
- 13hV. Lopez-Carrillo, A. M. Echavarren, J. Am. Chem. Soc. 2010, 132, 9292–9294;
- 13iA. Nishimura, M. Ohashi, S. Ogoshi, J. Am. Chem. Soc. 2012, 134, 15692–15695;
- 13jA. Abulimiti, A. Nishimura, M. Ohashi, S. Ogoshi, Chem. Lett. 2013, 42, 904–905;
- 13kK. Sakai, T. Kochi, F. Kakiuchi, Org. Lett. 2013, 15, 1024–1027;
- 13lR. Kumar, E. Tamai, A. Ohnishi, A. Nishimura, Y. Hoshimoto, M. Ohashi, S. Ogoshi, Synthesis 2016, 48, 2789–2794;
- 13mD. Kossler, F. G. Perrin, A. A. Suleymanov, G. Kiefer, R. Scopelliti, K. Severin, N. Cramer, Angew. Chem. Int. Ed. 2017, 56, 11490–11493; Angew. Chem. 2017, 129, 11648–11651;
- 13nH. Qin, J. Chen, K. Li, Z. He, Y. Zhou, B. Fan, Chem. Asian J. 2018, 13, 2431–2434.
- 14For a cobalt-catalyzed reductive [3+2] cycloaddition between enones and allenes, see: H.-T. Chang, T. T. Jayanth, C.-H. Cheng, J. Am. Chem. Soc. 2007, 129, 4166–4167.
- 15L. Shen, K. Zhao, K. Doitomi, R. Ganguly, Y.-X. Li, Z.-L. Shen, H. Hirao, T.-P. Loh, J. Am. Chem. Soc. 2017, 139, 13570–13578.
- 16CCDC 1879126 and 1879127 (3 fa, 8) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 17Q.-A. Chen, D. K. Kim, V. M. Dong, J. Am. Chem. Soc. 2014, 136, 3772–3775.