Kinetic Studies of Donor–Acceptor Cyclopropanes: The Influence of Structural and Electronic Properties on the Reactivity
M. Sc. Alexander Kreft
Technische Universität Braunschweig, Institut für Organische Chemie, Hagenring 30, 38106 Braunschweig, Germany
These authors contributed equally to this work.
Search for more papers by this authorM. Sc. Alexander Lücht
Technische Universität Braunschweig, Institut für Organische Chemie, Hagenring 30, 38106 Braunschweig, Germany
These authors contributed equally to this work.
Search for more papers by this authorProf. Dr. Jörg Grunenberg
Technische Universität Braunschweig, Institut für Organische Chemie, Hagenring 30, 38106 Braunschweig, Germany
Search for more papers by this authorProf. Dr. Peter G. Jones
Technische Universität Braunschweig, Institut für Anorganische und Analytische Chemie, Hagenring 30, 38106 Braunschweig, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Daniel B. Werz
Technische Universität Braunschweig, Institut für Organische Chemie, Hagenring 30, 38106 Braunschweig, Germany
Search for more papers by this authorM. Sc. Alexander Kreft
Technische Universität Braunschweig, Institut für Organische Chemie, Hagenring 30, 38106 Braunschweig, Germany
These authors contributed equally to this work.
Search for more papers by this authorM. Sc. Alexander Lücht
Technische Universität Braunschweig, Institut für Organische Chemie, Hagenring 30, 38106 Braunschweig, Germany
These authors contributed equally to this work.
Search for more papers by this authorProf. Dr. Jörg Grunenberg
Technische Universität Braunschweig, Institut für Organische Chemie, Hagenring 30, 38106 Braunschweig, Germany
Search for more papers by this authorProf. Dr. Peter G. Jones
Technische Universität Braunschweig, Institut für Anorganische und Analytische Chemie, Hagenring 30, 38106 Braunschweig, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Daniel B. Werz
Technische Universität Braunschweig, Institut für Organische Chemie, Hagenring 30, 38106 Braunschweig, Germany
Search for more papers by this authorDedicated to Professor Armin de Meijere on the occasion of his 80th birthday
Graphical Abstract
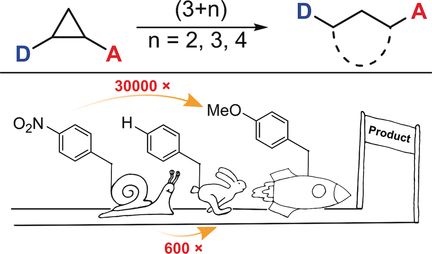
Who is faster? The reactivity of donor–acceptor cyclopropanes was investigated in (3+n) cycloaddition reactions with an aldehyde, a nitrone, and an isobenzofuran by NMR and in operando IR spectroscopy. The obtained reaction rates were compared with the structural and electronic properties of the donor–acceptor cyclopropanes.
Abstract
The kinetics of (3+2) cycloaddition reactions of 18 different donor–acceptor cyclopropanes with the same aldehyde were studied by in situ NMR spectroscopy. Increasing the electron density of the donor residue accelerates the reaction by a factor of up to 50 compared to the standard system (donor group=phenyl), whereas electron-withdrawing substituents slow down the reaction by a factor up to 660. This behavior is in agreement with the Hammett substituent parameter σ. The obtained rate constants from the (3+2) cycloadditions correlate well with data from additionally studied (3+n) cycloadditions with a nitrone (n=3) and an isobenzofuran (n=4). A comparison of the kinetic data with the bond lengths in the cyclopropane (obtained by X-ray diffraction and computation), or the 1H and 13C NMR shifts, revealed no correlation. However, the computed relaxed force constants of donor–acceptor cyclopropanes proved to be a good indicator for the reactivity of the three-membered ring.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201812880-sup-0001-misc_information.pdf17.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. de Meijere, Angew. Chem. Int. Ed. Engl. 1979, 18, 809; Angew. Chem. 1979, 91, 867.
- 2
- 2aC. Brückner, H.-U. Reissig, Angew. Chem. Int. Ed. Engl. 1985, 24, 588; Angew. Chem. 1985, 97, 578;
- 2bH.-U. Reissig, E. Hirsch, Angew. Chem. 1980, 19, 813; Angew. Chem. 1980, 92, 839;
- 2cE. Wenkert, M. E. Alonso, B. L. Buckwalter, K. J. Chou, J. Am. Chem. Soc. 1977, 99, 4778.
- 3
- 3aH. K. Grover, M. R. Emmett, M. A. Kerr, Org. Biomol. Chem. 2015, 13, 655;
- 3bR. A. Novikov, Y. V. Tomilov, Mendeleev Commun. 2015, 25, 1;
- 3cM. A. Cavitt, L. H. Phun, S. France, Chem. Soc. Rev. 2014, 43, 804;
- 3dT. F. Schneider, J. Kaschel, D. B. Werz, Angew. Chem. Int. Ed. 2014, 53, 5504; Angew. Chem. 2014, 126, 5608;
- 3eC. A. Carson, M. A. Kerr, Chem. Soc. Rev. 2009, 38, 3051;
- 3fF. De Simone, J. Waser, Synthesis 2009, 2009, 3353;
- 3gM. Yu, B. L. Pagenkopf, Tetrahedron 2005, 61, 321;
- 3hH.-U. Reissig, R. Zimmer, Chem. Rev. 2003, 103, 1151.
- 4
- 4aO. A. Ivanova, A. O. Chagarovskiy, A. N. Shumsky, V. D. Krasnobrov, I. I. Levina, I. V. Trushkov, J. Org. Chem. 2018, 83, 543;
- 4bA. Ortega, R. Manzano, U. Uria, L. Carrillo, E. Reyes, T. Tejero, P. Merino, J. L. Vicario, Angew. Chem. Int. Ed. 2018, 57, 8225; Angew. Chem. 2018, 130, 8357;
- 4cJ. Kaschel, C. D. Schmidt, M. Mumby, D. Kratzert, D. Stalke, D. B. Werz, Chem. Commun. 2013, 49, 4403;
- 4dC. D. Schmidt, J. Kaschel, T. F. Schneider, D. Kratzert, D. Stalke, D. B. Werz, Org. Lett. 2013, 15, 6098;
- 4eJ. Kaschel, T. F. Schneider, D. Kratzert, D. Stalke, D. B. Werz, Angew. Chem. Int. Ed. 2012, 51, 11153; Angew. Chem. 2012, 124, 11315;
- 4fT. F. Schneider, J. Kaschel, S. I. Awan, B. Dittrich, D. B. Werz, Chem. Eur. J. 2010, 16, 11276;
- 4gC. Brand, G. Rauch, M. Zanoni, B. Dittrich, D. B. Werz, J. Org. Chem. 2009, 74, 8779.
- 5
- 5aD. D. Borisov, R. A. Novikov, Y. V. Tomilov, Angew. Chem. Int. Ed. 2016, 55, 12233; Angew. Chem. 2016, 128, 12421;
- 5bT. Chidley, N. Vemula, C. A. Carson, M. A. Kerr, B. L. Pagenkopf, Org. Lett. 2016, 18, 2922;
- 5cJ. E. Curiel Tejeda, L. C. Irwin, M. A. Kerr, Org. Lett. 2016, 18, 4738;
- 5dL. K. B. Garve, M. Pawliczek, J. Wallbaum, P. G. Jones, D. B. Werz, Chem. Eur. J. 2016, 22, 521;
- 5eL. K. B. Garve, M. Petzold, P. G. Jones, D. B. Werz, Org. Lett. 2016, 18, 564;
- 5fA. Ghosh, S. Mandal, P. K. Chattaraj, P. Banerjee, Org. Lett. 2016, 18, 4940;
- 5gJ.-Q. Han, H.-H. Zhang, P.-F. Xu, Y.-C. Luo, Org. Lett. 2016, 18, 5212;
- 5hS. Racine, B. Hegedus, R. Scopelliti, J. Waser, Chem. Eur. J. 2016, 22, 11997;
- 5iJ. Sabbatani, N. Maulide, Angew. Chem. Int. Ed. 2016, 55, 6780; Angew. Chem. 2016, 128, 6892;
- 5jZ. Yuan, W. Wei, A. Lin, H. Yao, Org. Lett. 2016, 18, 3370;
- 5kR. A. Novikov, A. V. Tarasova, V. A. Korolev, E. V. Shulishov, V. P. Timofeev, Y. V. Tomilov, J. Org. Chem. 2015, 80, 8225;
- 5lH. Xu, J.-L. Hu, L. Wang, S. Liao, Y. Tang, J. Am. Chem. Soc. 2015, 137, 8006;
- 5mS. Chakrabarty, I. Chatterjee, B. Wibbeling, C. G. Daniliuc, A. Studer, Angew. Chem. Int. Ed. 2014, 53, 5964; Angew. Chem. 2014, 126, 6074;
- 5nW. D. Mackay, M. Fistikci, R. M. Carris, J. S. Johnson, Org. Lett. 2014, 16, 1626;
- 5oR. A. Novikov, A. V. Tarasova, V. A. Korolev, V. P. Timofeev, Y. V. Tomilov, Angew. Chem. Int. Ed. 2014, 53, 3187; Angew. Chem. 2014, 126, 3251;
- 5pS. Racine, F. de Nanteuil, E. Serrano, J. Waser, Angew. Chem. Int. Ed. 2014, 53, 8484; Angew. Chem. 2014, 126, 8624;
- 5qJ. Zhu, Y. Liang, L. Wang, Z.-B. Zheng, K. N. Houk, Y. Tang, J. Am. Chem. Soc. 2014, 136, 6900;
- 5rW. Zhu, J. Fang, Y. Liu, J. Ren, Z. Wang, Angew. Chem. Int. Ed. 2013, 52, 2032; Angew. Chem. 2013, 125, 2086.
- 6
- 6aA. O. Chagarovskiy, V. S. Vasin, V. V. Kuznetsov, O. A. Ivanova, V. B. Rybakov, A. N. Shumsky, N. N. Makhova, I. V. Trushkov, Angew. Chem. Int. Ed. 2018, 57, 10338; Angew. Chem. 2018, 130, 10495;
- 6bA. U. Augustin, M. Busse, P. G. Jones, D. B. Werz, Org. Lett. 2018, 20, 820;
- 6cY. Matsumoto, D. Nakatake, R. Yazaki, T. Ohshima, Chem. Eur. J. 2018, 24, 6062;
- 6dA. U. Augustin, M. Sensse, P. G. Jones, D. B. Werz, Angew. Chem. Int. Ed. 2017, 56, 14293; Angew. Chem. 2017, 129, 14481;
- 6eJ. Blom, A. Vidal-Albalat, J. Jørgensen, C. L. Barløse, K. S. Jessen, M. V. Iversen, K. A. Jørgensen, Angew. Chem. Int. Ed. 2017, 56, 11831; Angew. Chem. 2017, 129, 11993;
- 6fR. Dey, P. Banerjee, Org. Lett. 2017, 19, 304;
- 6gL. K. B. Garve, A. Kreft, P. G. Jones, D. B. Werz, J. Org. Chem. 2017, 82, 9235;
- 6hK. Mondal, S. C. Pan, Eur. J. Org. Chem. 2017, 534;
- 6iR. A. Novikov, A. V. Tarasova, D. A. Denisov, D. D. Borisov, V. A. Korolev, V. P. Timofeev, Y. V. Tomilov, J. Org. Chem. 2017, 82, 2724;
- 6jZ. Su, S. Qian, S. Xue, C. Wang, Org. Biomol. Chem. 2017, 15, 7878;
- 6kG. Sudhakar, S. K. Mahesh, S. P. B. Vemulapalli, J. B. Nanubolu, Org. Lett. 2017, 19, 4500;
- 6lK. Verma, P. Banerjee, Adv. Synth. Catal. 2017, 359, 3848;
- 6mZ.-H. Wang, H.-H. Zhang, D.-M. Wang, P.-F. Xu, Y.-C. Luo, Chem. Commun. 2017, 53, 8521;
- 6nL. K. G. Garve, P. G. Jones, D. B. Werz, Angew. Chem. Int. Ed. 2017, 56, 9226; Angew. Chem. 2017, 129, 9354.
- 7
- 7aS. Das, C. G. Daniliuc, A. Studer, Angew. Chem. Int. Ed. 2018, 57, 4053; Angew. Chem. 2018, 130, 4117;
- 7bK. L. Ivanov, S. I. Bezzubov, M. Y. Melnikov, E. M. Budynina, Org. Biomol. Chem. 2018, 16, 3897;
- 7cE. Richmond, V. D. Vuković, J. Moran, Org. Lett. 2018, 20, 574;
- 7dB. M. Trost, W.-J. Bai, C. Hohn, Y. Bai, J. J. Cregg, J. Am. Chem. Soc. 2018, 140, 6710;
- 7eS. V. Zaytsev, K. L. Ivanov, D. A. Skvortsov, S. I. Bezzubov, M. Y. Melnikov, E. M. Budynina, J. Org. Chem. 2018, 83, 8695;
- 7fS. Das, C. G. Daniliuc, A. Studer, Angew. Chem. Int. Ed. 2017, 56, 11554; Angew. Chem. 2017, 129, 11712;
- 7gA. Lücht, L. J. Patalag, A. U. Augustin, P. G. Jones, D. B. Werz, Angew. Chem. Int. Ed. 2017, 56, 10587; Angew. Chem. 2017, 129, 10723;
- 7hY.-C. Luo, H. Ma, X.-Q. Hu, P.-F. Xu, Org. Lett. 2017, 82, 1013;
- 7iH. Ma, X.-Q. Hu, Y.-C. Luo, P.-F. Xu, Org. Lett. 2017, 19, 6666;
- 7jJ. Wallbaum, L. K. B. Garve, P. G. Jones, D. B. Werz, Org. Lett. 2017, 19, 98;
- 7kT. Kaicharla, T. Roy, M. Thangaraj, R. G. Gonnade, A. T. Biju, Angew. Chem. Int. Ed. 2016, 55, 10061; Angew. Chem. 2016, 128, 10215;
- 7lK. L. Ivanov, E. V. Villemson, E. M. Budynina, O. A. Ivanova, I. V. Trushkov, M. Y. Melnikov, Chem. Eur. J. 2015, 21, 4975;
- 7mH.-P. Wang, H.-H. Zhang, X.-Q. Hu, P.-F. Xu, Y.-C. Luo, Eur. J. Org. Chem. 2015, 3486;
- 7nL. K. B. Garve, P. Barkawitz, P. G. Jones, D. B. Werz, Org. Lett. 2014, 16, 5804;
- 7oF. de Nanteuil, J. Loup, J. Waser, Org. Lett. 2013, 15, 3738;
- 7pS. M. Wales, M. M. Walker, J. S. Johnson, Org. Lett. 2013, 15, 2558;
- 7qM. R. Emmett, H. K. Grover, M. A. Kerr, J. Org. Chem. 2012, 77, 6634;
- 7rY.-Y. Zhou, L.-J. Wang, J. Li, X.-L. Sun, Y. Tang, J. Am. Chem. Soc. 2012, 134, 9066.
- 8
- 8aD. Perrotta, M.-M. Wang, J. Waser, Angew. Chem. Int. Ed. 2018, 57, 5120; Angew. Chem. 2018, 130, 5214;
- 8bL. K. A. Pilsl, T. Ertl, O. Reiser, Org. Lett. 2017, 19, 2754;
- 8cJ. Wallbaum, L. K. B. Garve, P. G. Jones, D. B. Werz, Chem. Eur. J. 2016, 22, 18756;
- 8dD.-C. Wang, M.-S. Xie, H.-M. Guo, G.-R. Qu, M.-C. Zhang, S.-L. You, Angew. Chem. Int. Ed. 2016, 55, 14111; Angew. Chem. 2016, 128, 14317;
- 8eY. Xia, L. Lin, F. Chang, Y. Liao, X. Liu, X. Feng, Angew. Chem. Int. Ed. 2016, 55, 12228; Angew. Chem. 2016, 128, 12416; Chem. 2016, 128, 12416;
- 8fC. Sparr, R. Gilmour, Angew. Chem. Int. Ed. 2011, 50, 8391; Angew. Chem. 2011, 123, 8541.
- 9
- 9aA. Kreft, P. G. Jones, D. B. Werz, Org. Lett. 2018, 20, 2059;
- 9bD. A. Denisov, R. A. Novikov, K. V. Potapov, V. A. Korolev, E. V. Shulishov, Y. V. Tomilov, ChemistrySelect 2016, 1, 6374;
- 9cT. F. Schneider, D. B. Werz, Org. Lett. 2011, 13, 1848.
- 10
- 10aV. Lehner, H. M. L. Davies, O. Reiser, Org. Lett. 2017, 19, 4722;
- 10bS. J. Gharpure, L. N. Nanda, M. K. Shukla, Org. Lett. 2014, 16, 6424;
- 10cF. de Simone, J. Gertsch, J. Waser, Angew. Chem. Int. Ed. 2010, 49, 5767; Angew. Chem. 2010, 122, 5903.
- 11In order to allow for a quantitative analysis of the 19F NMR data, complete relaxation had to be ensured. Before the experiments, the optimum repetition time of the pulse sequence was estimated by an inversion-recovery (T1) experiment. A repetition time of 19.2 s (relaxation delay 15.9 s, acquisition time 3.3 s, 30° 19F excitation pulse) was used.
- 12P. D. Pohlhaus, S. D. Sanders, A. T. Parsons, W. Li, J. S. Johnson, J. Am. Chem. Soc. 2008, 130, 8642.
- 13For a detailed error analysis, see the Supporting Information. The measured data depicted in the diagrams have been scaled to the same initial value for better clarity. Such a procedure has no influence on the rate constants.
- 14C. Hansch, A. Leo, R. W. Taft, Chem. Rev. 1991, 91, 165.
- 15CCDC 1877369–1877377 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 16P. Müller, D. Fernandez, Helv. Chim. Acta 1995, 78, 947.
- 17R. Talukdar, D. P. Tiwari, A. Saha, M. K. Ghorai, Org. Lett. 2014, 16, 3954.
- 18C. Perreault, S. R. Goudreau, L. E. Zimmer, A. B. Charette, Org. Lett. 2008, 10, 689.
- 19A. Ghanem, F. Lacrampe, V. Schurig, Helv. Chim. Acta 2005, 88, 216.
- 20M. P. Doyle, S. B. Davies, W. Hu, Org. Lett. 2000, 2, 1145.
- 21
- 21aG. Markopoulos, J. Grunenberg, Angew. Chem. Int. Ed. 2013, 52, 10648; Angew. Chem. 2013, 125, 10842;
- 21bJ. Grunenberg, Angew. Chem. Int. Ed. 2017, 56, 7288; Angew. Chem. 2017, 129, 7394.
- 22This statement only seems to be correct for para-substituted derivatives. When comparing the RFC with differently substituted aryl residues or the heteroatom donors, we found some clear deviations (see the Supporting Information). However, both steric effects and the Lewis acid might strongly influence the kinetics in these cases.
- 23I. S. Young, M. A. Kerr, Angew. Chem. Int. Ed. 2003, 42, 3023; Angew. Chem. 2003, 115, 3131.
- 24O. A. Ivanova, E. M. Budynina, Y. K. Grishin, I. V. Trushkov, P. V. Verteletskii, Angew. Chem. Int. Ed. 2008, 47, 1107; Angew. Chem. 2008, 120, 1123.
- 25The following IR bands were monitored:
 =1553 cm−1 (nitrone) and
=1553 cm−1 (nitrone) and  =768, 1495 cm−1 (isobenzofuran).
=768, 1495 cm−1 (isobenzofuran).