Facile Synthesis of Unsolvated Alkali Metal Octahydrotriborate Salts MB3H8 (M=K, Rb, and Cs), Mechanisms of Formation, and the Crystal Structure of KB3H8
Xi-Meng Chen
School of Chemistry and Chemical Engineering, Henan Key Laboratory of Boron Chemistry and Advanced Energy Materials, Henan Normal University, Xinxiang, Henan, 453007 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Nana Ma
School of Chemistry and Chemical Engineering, Henan Key Laboratory of Boron Chemistry and Advanced Energy Materials, Henan Normal University, Xinxiang, Henan, 453007 China
These authors contributed equally to this work.
Search for more papers by this authorXin-Ran Liu
School of Chemistry and Chemical Engineering, Henan Key Laboratory of Boron Chemistry and Advanced Energy Materials, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorChanggeng Wei
School of Chemistry and Chemical Engineering, Henan Key Laboratory of Boron Chemistry and Advanced Energy Materials, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorChong-Chao Cui
School of Chemistry and Chemical Engineering, Henan Key Laboratory of Boron Chemistry and Advanced Energy Materials, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorBu-La Cao
School of Chemistry and Chemical Engineering, Henan Key Laboratory of Boron Chemistry and Advanced Energy Materials, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Yanhui Guo
Department of Materials Science, Fudan University, Shanghai, 200433 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Lai-Sheng Wang
Department of Chemistry, Brown University, Providence, RI, 02912 USA
Search for more papers by this authorDr. Qinfen Gu
Australian Synchrotron (ANSTO), 800 Blackburn Rd, Clayton, 3168 VIC, Australia
Search for more papers by this authorCorresponding Author
Prof. Dr. Xuenian Chen
School of Chemistry and Chemical Engineering, Henan Key Laboratory of Boron Chemistry and Advanced Energy Materials, Henan Normal University, Xinxiang, Henan, 453007 China
College of Chemistry and Molecular Engineering, Zhengzhou University, Zhengzhou, Henan, 450001 China
Search for more papers by this authorXi-Meng Chen
School of Chemistry and Chemical Engineering, Henan Key Laboratory of Boron Chemistry and Advanced Energy Materials, Henan Normal University, Xinxiang, Henan, 453007 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Nana Ma
School of Chemistry and Chemical Engineering, Henan Key Laboratory of Boron Chemistry and Advanced Energy Materials, Henan Normal University, Xinxiang, Henan, 453007 China
These authors contributed equally to this work.
Search for more papers by this authorXin-Ran Liu
School of Chemistry and Chemical Engineering, Henan Key Laboratory of Boron Chemistry and Advanced Energy Materials, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorChanggeng Wei
School of Chemistry and Chemical Engineering, Henan Key Laboratory of Boron Chemistry and Advanced Energy Materials, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorChong-Chao Cui
School of Chemistry and Chemical Engineering, Henan Key Laboratory of Boron Chemistry and Advanced Energy Materials, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorBu-La Cao
School of Chemistry and Chemical Engineering, Henan Key Laboratory of Boron Chemistry and Advanced Energy Materials, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Yanhui Guo
Department of Materials Science, Fudan University, Shanghai, 200433 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Lai-Sheng Wang
Department of Chemistry, Brown University, Providence, RI, 02912 USA
Search for more papers by this authorDr. Qinfen Gu
Australian Synchrotron (ANSTO), 800 Blackburn Rd, Clayton, 3168 VIC, Australia
Search for more papers by this authorCorresponding Author
Prof. Dr. Xuenian Chen
School of Chemistry and Chemical Engineering, Henan Key Laboratory of Boron Chemistry and Advanced Energy Materials, Henan Normal University, Xinxiang, Henan, 453007 China
College of Chemistry and Molecular Engineering, Zhengzhou University, Zhengzhou, Henan, 450001 China
Search for more papers by this authorGraphical Abstract
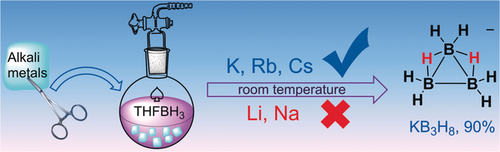
Sublime reactivity: Unsolvated MB3H8 (M=K, Rb, and Cs) salts were prepared by reactions of the respective pure alkali metals with THF⋅BH3 in high yield at room temperature, and the structure of unsolvated KB3H8 was elucidated. The proposed reaction mechanisms are based on experimental and theoretical results. It was found that the physical properties of the alkali metals play a crucial role.
Abstract
A facile synthesis of heavy alkali metal octahydrotriborates (MB3H8; M=K, Rb, and Cs) has been developed. It is simply based on reactions of the pure alkali metals with THF⋅BH3, does not require the use of electron carriers or the addition of other reaction media such as mercury, silica gel, or inert salts as for previous procedures, and delivers the desired products at room temperature in very high yields. However, no reactions were observed when pure Li or Na was used. The reaction mechanisms for the heavy alkali metals were investigated both experimentally and computationally. The low sublimation energies of K, Rb, and Cs were found to be key for initiation of the reactions. The syntheses can be carried out at room temperature because all of the elementary reaction steps have low energy barriers, whereas reactions of LiBH4/NaBH4 with THF⋅BH3 have to be carried out under reflux. The high stability and solubility of KB3H8 were examined, and a crystal structure thereof was obtained for the first time.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201812795-sup-0001-misc_information.pdf1.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C. W. Yoon, P. J. Carroll, L. G. Sneddon, J. Am. Chem. Soc. 2009, 131, 855–864.
- 2Z. Huang, X. Chen, T. Yisgedu, E. A. Meyers, S. G. Shore, J.-C. Zhao, Inorg. Chem. 2011, 50, 3738–3742.
- 3G. Kodama, R. W. Parry, J. C. Carter, J. Am. Chem. Soc. 1959, 81, 3534–3538.
- 4Z. Huang, M. Eagles, S. Porter, E. G. Sorte, B. Billet, R. L. Corey, M. S. Conradi, J.-C. Zhao, Dalton Trans. 2013, 42, 701–708.
- 5D. Y. Kim, Y. Yang, J. Y. Abelson, G. S. Girolami, Inorg. Chem. 2007, 46, 9060–9066.
- 6D. M. Goedde, G. S. Girolami, J. Am. Chem. Soc. 2004, 126, 12230–12231.
- 7Y. N. Shevchenko, A. V. Fesenko, T. M. Nazarova, O. K. Biryukovich, K. B. Yatsimirskii, U. M. Ogenko, Y. N. Sleasarenko, S. V. Naprasnaya, E. I. Larikov, R. A. Svitsyn, Otkrytiya Izobret. Prom. Obraztsy Tovarnye Znaki 1984, 31, 78.
- 8T. G. Hill, R. A. Godfroid, J. P. White, S. G. Shore, Inorg. Chem. 1991, 30, 2952–2954.
- 9A. Stock, E. Kuss, Chem. Ber. 1923, 56, 789–808.
- 10A. Stock, H. Laudenklos, Z. Anorg. Allg. Chem. 1936, 228, 178–192.
- 11A. Stock, E. Pohland, Chem. Ber. 1926, 59, 2210–2215.
- 12
- 12aZ. Huang, G. King, X. Chen, J. Hoy, T. Yisgedu, H. K. Lingam, S. G. Shore, P. M. Woodward, J.-C. Zhao, Inorg. Chem. 2010, 49, 8185–8187;
- 12bM. Chong, M. Matsuo, S. Orimo, T. Autrey, C. M. Jensen, Inorg. Chem. 2015, 54, 4120–4125;
- 12cM. Chong, A. Karkamkar, T. Autrey, S. Orimo, S. Jalisatgi, C. M. Jensen, Chem. Commun. 2011, 47, 1330–1332.
- 13
- 13aA. C. Dunbar, J. A. Macor, G. S. Girolami, Inorg. Chem. 2014, 53, 822–826;
- 13bM. Bürchner, A. M. T. Erle, H. Scherer, I. Krossing, Chem. Eur. J. 2012, 18, 2254–2262;
- 13cO. Palumbo, P. Nguyen, C. M. Jensen, A. Paolone, Int. J. Hydrogen Energy 2016, 41, 5986–5993.
- 14W. Chen, G. Wu, T. He, Z. Li, Z. Guo, H. Liu, Z. Huang, P. Chen, Int. J. Hydrogen Energy 2016, 41, 15471–15476.
- 15X. Zheng, Y. Yang, F. Zhao, F. Fang, Y. Guo, Chem. Commun. 2017, 53, 11083–11086.
- 16J. Huang, Y. Yan, A. Remhof, Y. Zhang, D. Rentsch, Y. S. Au, P. E. Jongh, F. Cuevas, L. Ouyang, M. Zhu, A. Züttel, Dalton Trans. 2016, 45, 3687–3690.
- 17D. F. Gaines, R. Schaeffer, F. Tebbe, Inorg. Chem. 1963, 2, 526–528.
- 18J. K. Olson, A. I. Boldyrev, Comput. Theor. Chem. 2011, 967, 1–4.
- 19
- 19aX.-M. Chen, N. Ma, Q.-F. Zhang, J. Wang, X. Feng, C. Wei, L.-S. Wang, J. Zhang, X. Chen, J. Am. Chem. Soc. 2018, 140, 6718–6726;
- 19bQ. Zhao, R. D. Dewhurst, H. Braunschweig, X. Chen, Angew. Chem. Int. Ed. 2019, https://doi.org/10.1002/anie.201809733; Angew. Chem. 2019, https://doi.org/10.1002/ange.201809733.
- 20R. Moury, A. Gigante, H. Hagemann, Int. J. Hydrogen Energy 2017, 42, 22417–22421.
- 21A. Yu. Bykov, K. Yu. Zhizhin, N. T. Kuznetsov, Russ. J. Inorg. Chem. 2014, 59, 1539–1555.
- 22H. Fu, X. Wang, Y. Shao, J. Chen, X. Zhang, H. Fu, J. Zheng, X. Li, Int. J. Hydrogen Energy 2016, 41, 384–391.
- 23M. J. Frisch et al., Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, 2013.
- 24R. A. Godfroid, T. G. Hill, T. P. Onak, S. G. Shore, J. Am. Chem. Soc. 1994, 116, 12107–12108.
- 25D. D. Wagman, W. H. Evans, V. B. Parker, R. H. Schumm, I. Halow, S. M. Bailey, K. L. Churney, R. L. Nutall, J. Phys. Chem. Ref. Data 1982, 11, Supplement No. 2.
- 26S. Pylypko, A. Zadick, M. Chatenet, P. Miele, M. Cretin, U. B. Demirci, J. Power Sources 2015, 286, 10–17.
- 27G. Kodama, R. W. Parry, J. Am. Chem. Soc. 1960, 82, 6250–6255.