Aromatic Azide Transformation on the Ag(111) Surface Studied by Scanning Probe Microscopy
Dr. Jack Hellerstedt
Institute of Physics, Academy of Sciences of the Czech Republic, Cukrovarnická 10, 16200 Prague 6, Czech Republic
These authors contributed equally to this work.
Search for more papers by this authorAleš Cahlík
Institute of Physics, Academy of Sciences of the Czech Republic, Cukrovarnická 10, 16200 Prague 6, Czech Republic
Regional Centre of Advanced Technologies and Materials, Faculty of Science, Palacký University, Šlechtitelû 27, 78371 Olomouc, Czech Republic
These authors contributed equally to this work.
Search for more papers by this authorDr. Oleksander Stetsovych
Institute of Physics, Academy of Sciences of the Czech Republic, Cukrovarnická 10, 16200 Prague 6, Czech Republic
National Institute for Materials Science (NIMS), 1-2-1 Sengen, Tsukuba, 305-0047 Japan
Search for more papers by this authorDr. Martin Švec
Institute of Physics, Academy of Sciences of the Czech Republic, Cukrovarnická 10, 16200 Prague 6, Czech Republic
Regional Centre of Advanced Technologies and Materials, Faculty of Science, Palacký University, Šlechtitelû 27, 78371 Olomouc, Czech Republic
Search for more papers by this authorDr. Tomoko K. Shimizu
National Institute for Materials Science (NIMS), 1-2-1 Sengen, Tsukuba, 305-0047 Japan
Search for more papers by this authorDr. Pingo Mutombo
Institute of Physics, Academy of Sciences of the Czech Republic, Cukrovarnická 10, 16200 Prague 6, Czech Republic
Search for more papers by this authorJiří Klívar
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorDr. Irena G. Stará
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorCorresponding Author
Dr. Pavel Jelínek
Institute of Physics, Academy of Sciences of the Czech Republic, Cukrovarnická 10, 16200 Prague 6, Czech Republic
Regional Centre of Advanced Technologies and Materials, Faculty of Science, Palacký University, Šlechtitelû 27, 78371 Olomouc, Czech Republic
Search for more papers by this authorCorresponding Author
Dr. Ivo Starý
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorDr. Jack Hellerstedt
Institute of Physics, Academy of Sciences of the Czech Republic, Cukrovarnická 10, 16200 Prague 6, Czech Republic
These authors contributed equally to this work.
Search for more papers by this authorAleš Cahlík
Institute of Physics, Academy of Sciences of the Czech Republic, Cukrovarnická 10, 16200 Prague 6, Czech Republic
Regional Centre of Advanced Technologies and Materials, Faculty of Science, Palacký University, Šlechtitelû 27, 78371 Olomouc, Czech Republic
These authors contributed equally to this work.
Search for more papers by this authorDr. Oleksander Stetsovych
Institute of Physics, Academy of Sciences of the Czech Republic, Cukrovarnická 10, 16200 Prague 6, Czech Republic
National Institute for Materials Science (NIMS), 1-2-1 Sengen, Tsukuba, 305-0047 Japan
Search for more papers by this authorDr. Martin Švec
Institute of Physics, Academy of Sciences of the Czech Republic, Cukrovarnická 10, 16200 Prague 6, Czech Republic
Regional Centre of Advanced Technologies and Materials, Faculty of Science, Palacký University, Šlechtitelû 27, 78371 Olomouc, Czech Republic
Search for more papers by this authorDr. Tomoko K. Shimizu
National Institute for Materials Science (NIMS), 1-2-1 Sengen, Tsukuba, 305-0047 Japan
Search for more papers by this authorDr. Pingo Mutombo
Institute of Physics, Academy of Sciences of the Czech Republic, Cukrovarnická 10, 16200 Prague 6, Czech Republic
Search for more papers by this authorJiří Klívar
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorDr. Irena G. Stará
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorCorresponding Author
Dr. Pavel Jelínek
Institute of Physics, Academy of Sciences of the Czech Republic, Cukrovarnická 10, 16200 Prague 6, Czech Republic
Regional Centre of Advanced Technologies and Materials, Faculty of Science, Palacký University, Šlechtitelû 27, 78371 Olomouc, Czech Republic
Search for more papers by this authorCorresponding Author
Dr. Ivo Starý
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorGraphical Abstract
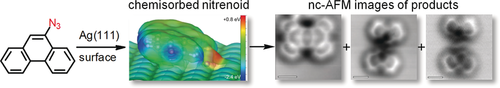
Coupling therapy with silver: Under ultrahigh vacuum, aryl azides generate an elusive chemisorbed nitrenoid intermediate on Ag(111). This intermediate can transform by forming covalent σ- and π-bonds via a formal nitrene insertion into a C−H bond, dimerisation, or hydrogenation. The structure of the transformation products was elucidated by high-resolution nc-AFM imaging supported by first-principle calculations.
Abstract
Chemical transformation of 9-azidophenanthrene on the Ag(111) surface was studied by nc-AFM in UHV. High-resolution imaging supported by first-principle calculations revealed the structure of the final products that originated from a common and elusive 9-phenanthryl nitrenoid intermediate chemisorbed on the Ag(111) surface. A formal nitrene insertion into the C−H bond along with its dimerisation and hydrogenation were identified as main reaction channels. Thus, the ability of aryl azides to form covalent σ- and π-bonds between their transformation products on a solid surface was demonstrated at a single-molecule level.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201812334-sup-0001-misc_information.pdf1.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1E. F. V. Scriven, K. Turnbull, Chem. Rev. 1988, 88, 297–368.
- 2
- 2aV. K. Tiwari, B. B. Mishra, K. B. Mishra, N. Mishra, A. S. Singh, X. Chen, Chem. Rev. 2016, 116, 3086–3240;
- 2bP. Thirumurugan, D. Matosiuk, K. Jozwiak, Chem. Rev. 2013, 113, 4905–4979.
- 3
- 3aJ.-F. Lutz, Angew. Chem. Int. Ed. 2007, 46, 1018–1025; Angew. Chem. 2007, 119, 1036–1043;
- 3bW. H. Binder, R. Sachsenhofer, Macromol. Rapid Commun. 2007, 28, 15–54.
- 4
- 4aM. Meldal, C. W. Tornøe, Chem. Rev. 2008, 108, 2952–3015;
- 4bJ. E. Moses, A. D. Moorhouse, Chem. Soc. Rev. 2007, 36, 1249–1262;
- 4cH. C. Kolb, M. G. Finn, K. B. Sharpless, Angew. Chem. Int. Ed. 2001, 40, 2004–2021;
10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5 CAS PubMed Web of Science® Google ScholarAngew. Chem. 2001, 113, 2056–2075.
- 5For recent reviews, see:
- 5aY. Park, Y. Kim, S. Chang, Chem. Rev. 2017, 117, 9247–9301;
- 5bB. Darses, R. Rodrigues, L. Neuville, M. Mazurais, P. Dauban, Chem. Commun. 2017, 53, 493–508;
- 5cK. Shin, H. Kim, S. Chang, Acc. Chem. Res. 2015, 48, 1040–1052;
- 5dD. Intrieri, P. Zardi, A. Caselli, E. Gallo, Chem. Commun. 2014, 50, 11440–11453;
- 5eJ. L. Roizen, M. E. Harvey, J. Du Bois, Acc. Chem. Res. 2012, 45, 911–922.
- 6
- 6aE. Smith, I. Collins, Future Med. Chem. 2015, 7, 159–183;
- 6bM. Hashimoto, Y. Hatanaka, Eur. J. Org. Chem. 2008, 2513–2523;
- 6cS. A. Fleming, Tetrahedron 1995, 51, 12479–12520.
- 7
- 7aS. Cenini, F. Ragaini, E. Gallo, A. Caselli, Curr. Org. Chem. 2011, 15, 1578–1592;
- 7bV. P. Semenov, A. N. Studenikov, A. A. Potekhin, Chem. Heterocycl. Compd. 1979, 15, 467–483.
10.1007/BF00773208 Google Scholar
- 8A. K. Schrock, G. B. Schuster, J. Am. Chem. Soc. 1984, 106, 5228–5234.
- 9
- 9aC. Wentrup, Acc. Chem. Res. 2011, 44, 393–404;
- 9b Organic Azides: Syntheses and Applications (Eds: ), Wiley, Chichester, 2010;
- 9cS. Bräse, C. Gil, K. Knepper, V. Zimmermann, Angew. Chem. Int. Ed. 2005, 44, 5188–5240; Angew. Chem. 2005, 117, 5320–5374;
- 9dM. S. Platz in Azides and Nitrenes (Ed.: ), Academic Press, San Diego, 1984, pp. 359–393.
10.1016/B978-0-12-633480-7.50011-0 Google Scholar
- 10D. Kvaskoff, P. Bednarek, L. George, S. Pankajakshan, C. Wentrup, J. Org. Chem. 2005, 70, 7947–7955.
- 11
- 11aE. Leyva, D. H. S. Chang, M. S. Platz, D. S. Watt, P. J. Crocker, K. Kawada, Photochem. Photobiol. 1991, 54, 329–333;
- 11bE. Leyva, M. S. Platz, G. Persy, J. Wirz, J. Am. Chem. Soc. 1986, 108, 3783–3790.
- 12
- 12aO. Díaz Arado, H. Mönig, H. Fuchs in On-Surface Synthesis (Ed.: ), Springer, Cham, 2016, pp. 101–114;
10.1007/978-3-319-26600-8_5 Google Scholar
- 12bF. Bebensee, C. Bombis, S.-R. Vadapoo, J. R. Cramer, F. Besenbacher, K. V. Gothelf, T. R. Linderoth, J. Am. Chem. Soc. 2013, 135, 2136–2139.
- 13
- 13aP. A. Held, H. Fuchs, A. Studer, Chem. Eur. J. 2017, 23, 5874–5892;
- 13bN. Pavliček, L. Gross, Nat. Rev. Chem. 2017, 1, 0005;
- 13c On-Surface Synthesis (Ed.: ), Springer, Cham, 2016;
- 13dX. Zhang, Q. Zeng, C. Wang, Nanoscale 2013, 5, 8269–8287;
- 13eJ. Méndez, M. F. López, J. A. Martín-Gago, Chem. Soc. Rev. 2011, 40, 4578–4590;
- 13fG. Franc, A. Gourdon, Phys. Chem. Chem. Phys. 2011, 13, 14283–14292;
- 13gA. Gourdon, Angew. Chem. Int. Ed. 2008, 47, 6950–6953; Angew. Chem. 2008, 120, 7056–7059.
- 14
- 14aL. Talirz, P. Shinde, D. Passerone, C. A. Pignedoli in On-Surface Synthesis (Ed.: ), Springer, Cham, 2016, pp. 237–268;
10.1007/978-3-319-26600-8_12 Google Scholar
- 14bL. Talirz, P. Ruffieux, R. Fasel, Adv. Mater. 2016, 28, 6222–6231;
- 14cA. Narita, X.-Y. Wang, X. Feng, K. Müllen, Chem. Soc. Rev. 2015, 44, 6616–6643;
- 14dL. Chen, Y. Hernandez, X. Feng, K. Müllen, Angew. Chem. Int. Ed. 2012, 51, 7640–7654; Angew. Chem. 2012, 124, 7758–7773;
- 14eJ. Cai, P. Ruffieux, R. Jaafar, M. Bieri, T. Braun, S. Blankenburg, M. Muoth, A. P. Seitsonen, M. Saleh, X. Feng, K. Müllen, R. Fasel, Nature 2010, 466, 470–473.
- 15
- 15aL. Dong, S. Wang, W. Wang, C. Chen, T. Lin, J. Adisoejoso, N. Lin in On-Surface Synthesis (Ed.: ), Springer, Cham, 2016, pp. 23–42;
10.1007/978-3-319-26600-8_2 Google Scholar
- 15bJ. A. Martín-Gago, A. L. Pinardi, J. I. Martínez in On-Surface Synthesis (Ed.: ), Springer, Cham, 2016, pp. 43–84.
10.1007/978-3-319-26600-8_3 Google Scholar
- 16
- 16aM. Kittelmann, R. Lindner, A. Kühnle in On-Surface Synthesis (Ed.: ), Springer, Cham, 2016, pp. 181–198;
10.1007/978-3-319-26600-8_9 Google Scholar
- 16bM. Kittelmann, P. Rahe, M. Nimmrich, C. M. Hauke, A. Gourdon, A. Kühnle, ACS Nano 2011, 5, 8420–8425;
- 16cM. Abel, S. Clair, O. Ourdjini, M. Mossoyan, L. Porte, J. Am. Chem. Soc. 2011, 133, 1203–1205.
- 17J. N. Denis, A. Krief, Tetrahedron 1979, 35, 2901–2903.
- 18M. Mamada, C. Pérez-Bolívar, D. Kumaki, N. A. Esipenko, S. Tokito, P. Anzenbacher, Jr., Chem. Eur. J. 2014, 20, 11835–11846.
- 19Commercially available; for recent preparations, see:
- 19aS. Voth, J. W. Hollett, J. A. McCubbin, J. Org. Chem. 2015, 80, 2545–2553;
- 19bC. W. Cheung, D. S. Surry, S. L. Buchwald, Org. Lett. 2013, 15, 3734–3737.
- 20D. Fujita, H. Sugimoto, Y. Shiota, Y. Morimoto, K. Yoshizawa, S. Itoh, Chem. Commun. 2017, 53, 4849–4852.
- 21K. T. Wong, J. T. Tanskanen, S. F. Bent, Langmuir 2013, 29, 15842–15850.
- 22L. Maestre, R. Dorel, Ó. Pablo, I. Escofet, W. M. C. Sameera, E. Álvarez, F. Maseras, M. M. Díaz-Requejo, A. M. Echavarren, P. J. Pérez, J. Am. Chem. Soc. 2017, 139, 2216–2223.
- 23S.-M. Au, J.-S. Huang, W.-Y. Yu, W.-H. Fung, C.-M. Che, J. Am. Chem. Soc. 1999, 121, 9120–9132.
- 24K. Shudo, K. Okamoto, Chem. Pharm. Bull. 1976, 24, 1013–1015.
- 25S. Kawai, K. Takahashi, S. Ito, R. Pawlak, T. Meier, P. Spijker, F. F. Canova, J. Tracey, K. Nozaki, A. S. Foster, E. Meyer, ACS Nano 2017, 11, 8122–8130.
- 26B. Nay, E. F. V. Scriven, H. Suschitzky, D. R. Thomas, J. Chem. Soc. Perkin Trans. 1 1980, 611–613.
Citing Literature
February 18, 2019
Pages 2266-2271