Paintable Room-Temperature Phosphorescent Liquid Formulations of Alkylated Bromonaphthalimide
Goudappagouda
Organic Chemistry Division, National Chemical Laboratory (CSIR-NCL), Dr. Homi Bhabha Road, Pune-, 411008 India
Academy of Scientific and Innovative Research (AcSIR), New Delhi, India
Search for more papers by this authorAnanthakrishnan Manthanath
Amrita Vishwa Vidyapeetham University, Amritapuri, Kerala-, 690525 India
Search for more papers by this authorVivek Chandrakant Wakchaure
Organic Chemistry Division, National Chemical Laboratory (CSIR-NCL), Dr. Homi Bhabha Road, Pune-, 411008 India
Academy of Scientific and Innovative Research (AcSIR), New Delhi, India
Search for more papers by this authorKayaramkodath Chandran Ranjeesh
Organic Chemistry Division, National Chemical Laboratory (CSIR-NCL), Dr. Homi Bhabha Road, Pune-, 411008 India
Academy of Scientific and Innovative Research (AcSIR), New Delhi, India
Search for more papers by this authorTamal Das
Academy of Scientific and Innovative Research (AcSIR), New Delhi, India
Physical and Materials Chemistry Division, National Chemical Laboratory (CSIR-NCL), Dr. Homi Bhabha Road, Pune-, 411008 India
Search for more papers by this authorKumar Vanka
Academy of Scientific and Innovative Research (AcSIR), New Delhi, India
Physical and Materials Chemistry Division, National Chemical Laboratory (CSIR-NCL), Dr. Homi Bhabha Road, Pune-, 411008 India
Search for more papers by this authorTakashi Nakanishi
Frontier Molecules Group, International Centre for Materials Nanoarchitectonics (WPI-MANA), National Institute for Materials Science (NIMS), 1-1 Namiki, Tsukuba, 305-0044 Japan
Search for more papers by this authorCorresponding Author
Sukumaran Santhosh Babu
Organic Chemistry Division, National Chemical Laboratory (CSIR-NCL), Dr. Homi Bhabha Road, Pune-, 411008 India
Academy of Scientific and Innovative Research (AcSIR), New Delhi, India
Search for more papers by this authorGoudappagouda
Organic Chemistry Division, National Chemical Laboratory (CSIR-NCL), Dr. Homi Bhabha Road, Pune-, 411008 India
Academy of Scientific and Innovative Research (AcSIR), New Delhi, India
Search for more papers by this authorAnanthakrishnan Manthanath
Amrita Vishwa Vidyapeetham University, Amritapuri, Kerala-, 690525 India
Search for more papers by this authorVivek Chandrakant Wakchaure
Organic Chemistry Division, National Chemical Laboratory (CSIR-NCL), Dr. Homi Bhabha Road, Pune-, 411008 India
Academy of Scientific and Innovative Research (AcSIR), New Delhi, India
Search for more papers by this authorKayaramkodath Chandran Ranjeesh
Organic Chemistry Division, National Chemical Laboratory (CSIR-NCL), Dr. Homi Bhabha Road, Pune-, 411008 India
Academy of Scientific and Innovative Research (AcSIR), New Delhi, India
Search for more papers by this authorTamal Das
Academy of Scientific and Innovative Research (AcSIR), New Delhi, India
Physical and Materials Chemistry Division, National Chemical Laboratory (CSIR-NCL), Dr. Homi Bhabha Road, Pune-, 411008 India
Search for more papers by this authorKumar Vanka
Academy of Scientific and Innovative Research (AcSIR), New Delhi, India
Physical and Materials Chemistry Division, National Chemical Laboratory (CSIR-NCL), Dr. Homi Bhabha Road, Pune-, 411008 India
Search for more papers by this authorTakashi Nakanishi
Frontier Molecules Group, International Centre for Materials Nanoarchitectonics (WPI-MANA), National Institute for Materials Science (NIMS), 1-1 Namiki, Tsukuba, 305-0044 Japan
Search for more papers by this authorCorresponding Author
Sukumaran Santhosh Babu
Organic Chemistry Division, National Chemical Laboratory (CSIR-NCL), Dr. Homi Bhabha Road, Pune-, 411008 India
Academy of Scientific and Innovative Research (AcSIR), New Delhi, India
Search for more papers by this authorIn memory of Helmuth Möhwald
Graphical Abstract
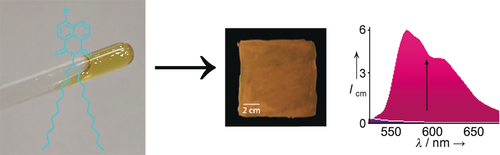
The room-temperature phosphorescence features of a solvent-free organic liquid phosphor in air were explored. Doping of the phosphor with carbonyl guests resulted in enhanced phosphorescence, and hence a large-area paintable phosphorescent liquid composite with improved lifetime and quantum yield was developed.
Abstract
Organic phosphors have been widely explored with an understanding that crystalline molecular ordering is a requisite for enhanced intersystem crossing. In this context, we explored the room-temperature phosphorescence features of a solvent-free organic liquid phosphor in air. While alkyl chain substitution varied the physical states of the bromonaphthalimides, the phosphorescence remained unaltered for the solvent-free liquid in air. As the first report, a solvent-free liquid of a long swallow-tailed bromonaphthalimide exhibits room-temperature phosphorescence in air. Doping of the phosphor with carbonyl guests resulted in enhanced phosphorescence, and hence a large-area paintable phosphorescent liquid composite with improved lifetime and quantum yield was developed.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201811834-sup-0001-misc_information.pdf4.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aX. Yang, G. Zhou, W.-Y. Wong, Chem. Soc. Rev. 2015, 44, 8484;
- 1bM. Baroncini, G. Bergamini, P. Ceroni, Chem. Commun. 2017, 53, 2081;
- 1cA. Forni, E. Lucenti, C. Botta, E. Cariati, J. Mater. Chem. C 2018, 6, 4603.
- 2
- 2aR. Kabe, N. Notsuka, K. Yoshida, C. Adachi, Adv. Mater. 2016, 28, 655;
- 2bG. Zhang, G. M. Palmer, M. W. Dewhirst, C. L. Fraser, Nat. Mater. 2009, 8, 747.
- 3
- 3aY. Gong, G. Chen, Q. Peng, W. Z. Yuan, Y. Xie, S. Li, Y. Zhang, B. Z. Tang, Adv. Mater. 2015, 27, 6195;
- 3bY. Shoji, Y. Ikabata, Q. Wang, D. Nemoto, A. Sakamoto, N. Tanaka, J. Seino, H. Nakai, T. Fukushima, J. Am. Chem. Soc. 2017, 139, 2728;
- 3cJ. Yang, Z. Ren, Z. Xie, Y. Liu, C. Wang, Y. Xie, Q. Peng, B. Xu, W. Tian, F. Zhang, Z. Chi, Q. Li, Z. Li, Angew. Chem. Int. Ed. 2017, 56, 880; Angew. Chem. 2017, 129, 898;
- 3dV. C. Wakchaure, K. C. Ranjeesh, Goudappagouda, T. Das, K. Vanka, R. Gonnade, S. S. Babu, Chem. Commun. 2018, 54, 6028.
- 4
- 4aO. Bolton, K. Lee, H.-J. Kim, K. Y. Lin, J. Kim, Nat. Chem. 2011, 3, 205;
- 4bZ. Mao, Z. Yang, Y. Mu, Y. Zhang, Y.-F. Wang, Z. Chi, C.-C. Lo, S. Liu, A. Lien, J. Xu, Angew. Chem. Int. Ed. 2015, 54, 6270; Angew. Chem. 2015, 127, 6368;
- 4cZ. An, C. Zheng, Y. Tao, R. Chen, H. Shi, T. Chen, Z. Wang, H. Li, R. Deng, X. Liu, W. Huang, Nat. Mater. 2015, 14, 685;
- 4dS. M. A. Fateminia, Z. Mao, S. Xu, Z. Yang, Z. Chi, B. Liu, Angew. Chem. Int. Ed. 2017, 56, 12160; Angew. Chem. 2017, 129, 12328.
- 5
- 5aM. S. Kwon, D. Lee, S. Seo, J. Jung, J. Kim, Angew. Chem. Int. Ed. 2014, 53, 11177; Angew. Chem. 2014, 126, 11359;
- 5bH. Mieno, R. Kabe, N. Notsuka, M. D. Allendorf, C. Adachi, Adv. Opt. Mater. 2016, 4, 1015.
- 6
- 6aH. Chen, X. Ma, S. Wu, H. Tian, Angew. Chem. Int. Ed. 2014, 53, 14149; Angew. Chem. 2014, 126, 14373;
- 6bL. Xu, L. Zou, H. Chen, X. Ma, Dyes Pigm. 2017, 142, 300.
- 7
- 7aS. S. Babu, T. Nakanishi, Chem. Commun. 2013, 49, 9373;
- 7bS. S. Babu, Phys. Chem. Chem. Phys. 2015, 17, 3950;
- 7cA. Ghosh, T. Nakanishi, Chem. Commun. 2017, 53, 10344.
- 8
- 8aS. S. Babu, J. Aimi, H. Ozawa, N. Shirahata, A. Saeki, S. Seki, A. Ajayaghosh, H. Möhwald, T. Nakanishi, Angew. Chem. Int. Ed. 2012, 51, 3391; Angew. Chem. 2012, 124, 3447;
- 8bS. S. Babu, M. J. Hollamby, J. Aimi, H. Ozawa, A. Saeki, S. Seki, K. Kobayashi, K. Hagiwara, M. Yoshizawa, H. Möhwald, T. Nakanishi, Nat. Commun. 2013, 4, 1969;
- 8cP. Duan, N. Yanai, N. Kimizuka, J. Am. Chem. Soc. 2013, 135, 19056;
- 8dF. Lu, T. Takaya, K. Iwata, I. Kawamura, A. Saeki, M. Ishii, K. Nagura, T. Nakanishi, Sci. Rep. 2017, 7, 3416;
- 8eT. Machida, R. Taniguchi, T. Oura, K. Sada, K. Kokado, Chem. Commun. 2017, 53, 2378.
- 9
- 9aN. Kobayashi, T. Kasahara, T. Edura, J. Oshima, R. Ishimatsu, M. Tsuwaki, T. Imato, S. Shoji, J. Mizuno, Sci. Rep. 2015, 5, 14822;
- 9bT. Kasahara, S. Matsunami, T. Edura, R. Ishimatsu, J. Oshima, M. Tsuwaki, T. Imato, S. Shoji, C. Adachi, J. Mizuno, Sens. Actuators B 2015, 207, 481;
- 9cA. S. D. Sandanayaka, L. Zhao, D. Pitrat, J. C. Mulatier, T. Matsushima, C. Andraud, J. H. Kim, J. C. Ribierre, C. Adachi, Appl. Phys. Lett. 2016, 108, 223301.
- 10
- 10aX. Zhan, A. Facchetti, S. Barlow, T. J. Marks, M. A. Ratner, M. R. Wasielewski, S. R. Marder, Adv. Mater. 2011, 23, 268;
- 10bS.-L. Suraru, F. Würthner, Angew. Chem. Int. Ed. 2014, 53, 7428; Angew. Chem. 2014, 126, 7558;
- 10cM. Al Kobaisi, S. V. Bhosale, K. Latham, A. M. Raynor, S. V. Bhosale, Chem. Rev. 2016, 116, 11685;
- 10dA. Sarkar, S. Dhiman, A. Chalishazar, S. J. George, Angew. Chem. Int. Ed. 2017, 56, 13767; Angew. Chem. 2017, 129, 13955.
- 11
- 11aB. Ventura, A. Bertocco, D. Braga, L. Catalano, S. d'Agostino, F. Grepioni, P. Taddei, J. Phys. Chem. C 2014, 118, 18646;
- 11bX. Chen, C. Xu, T. Wang, C. Zhou, J. Du, Z. Wang, H. Xu, T. Xie, G. Bi, J. Jiang, X. Zhang, J. N. Demas, C. O. Trindle, Y. Luo, G. Zhang, Angew. Chem. Int. Ed. 2016, 55, 9872; Angew. Chem. 2016, 128, 10026;
- 11cH. Chen, L. Xu, X. Ma, H. Tian, Polym. Chem. 2016, 7, 3989;
- 11dH. Chen, X. Yao, X. Ma, H. Tian, Adv. Opt. Mater. 2016, 4, 1397.
- 12G. Zhang, J. Chen, S. J. Payne, S. E. Kooi, J. N. Demas, C. L. Fraser, J. Am. Chem. Soc. 2007, 129, 8942.
- 13CCDC 1873219 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 14J. Wei, B. Liang, R. Duan, Z. Cheng, C. Li, T. Zhou, Y. Yi, Y. Wang, Angew. Chem. Int. Ed. 2016, 55, 15589; Angew. Chem. 2016, 128, 15818.
- 15
- 15aG. R. Desiraju, P. S. Ho, L. Kloo, A. C. Legon, R. Marquardt, P. Metrangolo, P. Politzer, G. Resnati, K. Rissanen, Pure Appl. Chem. 2013, 85, 1711;
- 15bG. Cavallo, P. Metrangolo, R. Milani, T. Pilati, A. Priimagi, G. Resnati, G. Terraneo, Chem. Rev. 2016, 116, 2478.