Quadruply Twisted Hückel-Aromatic Dodecaphyrin
Takanori Soya
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorDr. Hirotaka Mori
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Atsuhiro Osuka
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorTakanori Soya
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorDr. Hirotaka Mori
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Atsuhiro Osuka
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorGraphical Abstract
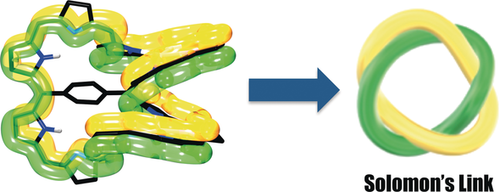
All in a twist: π-Conjugated macrocycles possessing a quadruply twisted topology were realized by conformational fixation using the insertion of a 1,4-phenylene bridge and PdII metalation. The syn-isomer of bis-PdII complexes displays aromatic character, and is thus identified as the first example of quadruply twisted Hückel aromatic molecule.
Abstract
Molecular topology of π-conjugated circuits becomes increasingly important in the chemistry of aromatic and antiaromatic compounds. meso-Pentafluorophenyl-substituted 5,35-(1,4-phenylene)bridged [56]dodecaphyrin was synthesized by condensation of 1,4-phenylene-bridged dicarbinol dimer and 5,10,15-tris-(pentafluorophenyl)tetrapyrrane followed by oxidation with DDQ and was oxidized to its [54]- and [52]congeners in a stepwise manner. Metalation of the [52]dodecaphyrin with Pd2(dba)3 gave two bis-PdII complexes that are isomers of metalation sites: anti and syn with regard to the 1,4-phenylene bridge. The anti-isomer was easily oxidized to its N-fused form, a quadruply twisted non-aromatic or weakly aromatic macrocycle. On the other hand, the syn-isomer was revealed to be the first example of Hückel aromatic molecule with a quadruply twisted structure.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201811433-sup-0001-misc_information.pdf13.4 MB | Supplementary |
| anie201811433-sup-0001-Movie_13.mp441.6 MB | Supplementary |
| anie201811433-sup-0001-Movie_25.mp443.1 MB | Supplementary |
| anie201811433-sup-0001-Movie_3.mp450.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Recent reviews for aromaticity:
- 1aP. v. R. Schleyer, Chem. Rev. 2001, 101, 1115;
- 1bP. v. R. Schleyer, Chem. Rev. 2005, 105, 3433;
- 1cN. Martín, L. T. Scott, Chem. Soc. Rev. 2015, 44, 6389.
- 2
- 2aA. B. McEvan, P. v. R. Schleyer, J. Org. Chem. 1986, 51, 4357;
- 2bN. Toriumi, A. Muranaka, E. Kayahara, S. Yamago, M. Uchiyama, J. Am. Chem. Soc. 2015, 137, 82;
- 2cM. D. Peeks, T. D. W. Claridge, H. L. Anderson, Nature 2017, 541, 200.
- 3
- 3aC. Corminboeuf, P. v. R. Schleyer, P. Warner, Org. Lett. 2007, 9, 3263;
- 3bD. E. Bean, P. W. Fowler, Org. Lett. 2008, 10, 5573;
- 3cR. Nozawa, H. Tanaka, W.-Y. Cha, Y. Hong, I. Hisaki, S. Shimizu, J.-Y. Shin, T. Kowalczyk, S. Irle, D. Kim, H. Shinokubo, Nat. Commun. 2016, 7, 13620.
- 4Reviews for twisted π-conjugated molecules:
- 4aH. S. Rzepa, Chem. Rev. 2005, 105, 3697;
- 4bR. Herges, Chem. Rev. 2006, 106, 4820;
- 4cG. R. Schaller, R. Herges, Chem. Commun. 2013, 49, 1254.
- 5The first synthetic examples of Möbius aromatic hydrocarbons:
- 5aD. Ajami, O. Oeckeler, A. Simon, R. Herges, Nature 2003, 426, 819;
- 5bD. Ajami, K. Hess, F. Köhler, C. Näther, O. Oeckeler, A. Simon, C. Yamamoto, Y. Okamoto, R. Herges, Chem. Eur. J. 2006, 12, 5434.
- 6
- 6aH. S. Rzepa, Chem. Commun. 2005, 5220;
- 6bH. S. Rzepa, Org. Lett. 2005, 7, 4637;
- 6cP. W. Fowler, H. S. Rzepa, Phys. Chem. Chem. Phys. 2006, 8, 1775;
- 6dH. S. Rzepa, Org. Lett. 2008, 10, 949;
- 6eA. R. Mohebbi, E.-K. Mucke, G. R. Schaller, F. Köhler, F. D. Sönnichsen, L. Ernst, C. Näther, R. Herges, Chem. Eur. J. 2010, 16, 7767.
- 7S. M. Rappaport, H. S. Rzepa, J. Am. Chem. Soc. 2008, 130, 7613.
- 8Triply twisted π-conjugated macrocycles:
- 8aG. R. Schaller, F. Topić, K. Rissanen, Y. Okamoto, J. Shen, R. Herges, Nat. Chem. 2014, 6, 608;
- 8bE. Wang, Z. He, E. Zhao, L. Meng, C. Schütt, J. W. Y. Lam, H. H. Y. Sung, I. D. Williams, X. Huang, R. Herges, B. Z. Tang, Chem. Eur. J. 2015, 21, 11707.
- 9
- 9aJ. L. Sessler, D. Seidel, Angew. Chem. Int. Ed. 2003, 42, 5134; Angew. Chem. 2003, 115, 5292;
- 9bM. Stępień, N. Sprutta, L. Latos-Grażyński, Angew. Chem. Int. Ed. 2011, 50, 4288; Angew. Chem. 2011, 123, 4376;
- 9cS. Saito, A. Osuka, Angew. Chem. Int. Ed. 2011, 50, 4342; Angew. Chem. 2011, 123, 4432;
- 9dA. Osuka, S. Saito, Chem. Commun. 2011, 47, 4330;
- 9eT. Tanaka, A. Osuka, Chem. Rev. 2017, 117, 2584;
- 9fB. Szyszko, M. J. Białek, E. Pacholska-Dudziak, L. Latos-Grażyński, Chem. Rev. 2017, 117, 2839.
- 10Quadruply twisted π-conjugated macrocycles:
- 10aS. Shimizu, N. Aratani, A. Osuka, Chem. Eur. J. 2006, 12, 4909;
- 10bT. Yoneda, T. Soya, S. Neya, A. Osuka, Chem. Eur. J. 2016, 22, 14518.
- 11Internally bridged expanded porphyrins have been explored in the last decade:
- 11aV. G. Anand, S. Saito, S. Shimizu, A. Osuka, Angew. Chem. Int. Ed. 2005, 44, 7244; Angew. Chem. 2005, 117, 7410;
- 11bM. Suzuki, A. Osuka, J. Am. Chem. Soc. 2007, 129, 464;
- 11cG. Karthik, M. Sneha, V. P. Raja, J. M. Lim, D. Kim, A. Srinivasan, T. K. Chandrashekar, Chem. Eur. J. 2013, 19, 1886;
- 11dH. Mori, J. M. Lim, D. Kim, A. Osuka, Angew. Chem. Int. Ed. 2013, 52, 12997; Angew. Chem. 2013, 125, 13235;
- 11eJ. M. Lim, K. Ganesan, Y. M. Sung, A. Srinivasan, T. K. Chandrashekar, D. Kim, Chem. Commun. 2014, 50, 1837;
- 11fY. M. Sung, J. Oh, W. Kim, H. Mori, A. Osuka, D. Kim, J. Am. Chem. Soc. 2015, 137, 11856;
- 11gG. Karthik, W.-Y. Cha, A. Ghosh, T. Kim, A. Srinivasan, D. Kim, T. K. Chandrashekar, Chem. Asian J. 2016, 11, 1447;
- 11hT. Soya, H. Mori, Y. Hong, Y. H. Koo, D. Kim, A. Osuka, Angew. Chem. Int. Ed. 2017, 56, 3232; Angew. Chem. 2017, 129, 3280;
- 11iW.-Y. Cha, T. Kim, A. Ghosh, Z. Zhang, X.-S. Ke, R. Ali, V. M. Lynch, J. Jung, W. Kim, S. Lee, S. Fukuzumi, J. S. Park, J. L. Sessler, T. K. Chandrashekar, D. Kim, Nat. Chem. 2017, 9, 1243;
- 11jM. J. Białek, L. Latos-Grażyński, Chem. Commun. 2018, 54, 1837.
- 12
- 12aCrystallographic data for 3: C126H36F50N12⋅8(C6H6)⋅2(C6), Mr=3436.66; triclinic; space group P
 (No.2), a=14.177(2), b=16.712(4), c=17.278(3) Å; α=106.043(5), β=104.1545(3), γ=97.629(7)°; V=3725.5(12) Å3; ρcalcd=1.174 g cm−3; Z=1; R1=0.0531 [I>2.0σ(I)], wR2=0.1666 (all data), GOF=1.052;
(No.2), a=14.177(2), b=16.712(4), c=17.278(3) Å; α=106.043(5), β=104.1545(3), γ=97.629(7)°; V=3725.5(12) Å3; ρcalcd=1.174 g cm−3; Z=1; R1=0.0531 [I>2.0σ(I)], wR2=0.1666 (all data), GOF=1.052;
- 12bCrystallographic data for 5: C126H32F50N12⋅4(CHCl3), Mr=3104.82; monoclinic; space group C2/c (No.15), a=63.06(3), b=20.5623(10), c=23.476(10) Å; β=91.853(12)°; V=30424(19) Å3; ρcalcd=1.356 g cm−3; Z=8; R1=0.0865 [I>2.0σ(I)], wR2=0.2749 (all data), GOF=1.009;
- 12cCrystallographic data for 6-fused: 2(C126H28F50N12Pd2)⋅6.822(C6H6)⋅2(C2O), Mr=6379.24; triclinic; space group P
 (No.2), a=19.2854(13), b=28.8466(12), c=30.6271(18) Å; α=108.8038(10), β=108.136(14), γ=103.865(15)°; V=14191(2) Å3; ρcalcd=1.493 g cm−3; Z=2; R1=0.0703 [I>2.0σ(I)], wR2=0.2190 (all data), GOF=1.009;
(No.2), a=19.2854(13), b=28.8466(12), c=30.6271(18) Å; α=108.8038(10), β=108.136(14), γ=103.865(15)°; V=14191(2) Å3; ρcalcd=1.493 g cm−3; Z=2; R1=0.0703 [I>2.0σ(I)], wR2=0.2190 (all data), GOF=1.009;
- 12dCrystallographic data for 7: C126H30F50N12Pd2⋅3(C6O)⋅6(CCl4), Mr=4061.46; monoclinic; space group C2/c (No.15), a=26.6753(4), b=29.1994(4), c=42.9903(6) Å; β=105.940(2)°; V=32197.7(9) Å3; ρcalcd=1.676 g cm−3; Z=8; R1=0.1759 [I>2.0σ(I)], wR2=0.4388 (all data), GOF=1.081;
- 12eCCDC 1868904 (3), 1868905 (5), 1868906 (6-fused), and 1868907 (7) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre. The contributions to the scattering arising from the presence of the disordered solvents in the crystals of 5, 6-fused, and 7 were removed by use of the utility SQUEEZE in the PLATON software package; see: A. L. Spek, PLATON, A Multipurpose Crystallographic Tool, Utrecht University, Utrecht, The Netherlands, 2005; P. van der Sluis, A. L. Spek, Acta Crystallogr. Sect. A 1990, 46, 194.
- 13T. Yoneda, Y. M. Sung, J. M. Lim, D. Kim, A. Osuka, Angew. Chem. Int. Ed. 2014, 53, 13169; Angew. Chem. 2014, 126, 13385.
- 14
- 14aP. v. R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao, N. J. R. v. E. Hommes, J. Am. Chem. Soc. 1996, 118, 6317;
- 14bZ. Chen, C. S. Wannere, C. Corminboeuf, R. Puchta, P. v. R. Schleyer, Chem. Rev. 2005, 105, 3842.
- 15
- 15aR. Herges, D. Geuenich, J. Phys. Chem. A 2001, 105, 3214;
- 15bD. Geuenich, K. Hess, F. Köhler, R. Herges, Chem. Rev. 2005, 105, 3758.