Boron(III) Carbazosubphthalocyanines: Core-Expanded Antiaromatic Boron(III) Subphthalocyanine Analogues
Joseph Y. M. Chan
Department of Chemistry, The Chinese University of Hong Kong, Shatin, N.T., Hong Kong, China
Search for more papers by this authorTakahiro Kawata
Department of Chemistry and Materials, Faculty of Textile Science and Technology, Shinshu University, Ueda, 386-8567 Japan
Search for more papers by this authorCorresponding Author
Prof. Nagao Kobayashi
Department of Chemistry and Materials, Faculty of Textile Science and Technology, Shinshu University, Ueda, 386-8567 Japan
Search for more papers by this authorCorresponding Author
Prof. Dennis K. P. Ng
Department of Chemistry, The Chinese University of Hong Kong, Shatin, N.T., Hong Kong, China
Search for more papers by this authorJoseph Y. M. Chan
Department of Chemistry, The Chinese University of Hong Kong, Shatin, N.T., Hong Kong, China
Search for more papers by this authorTakahiro Kawata
Department of Chemistry and Materials, Faculty of Textile Science and Technology, Shinshu University, Ueda, 386-8567 Japan
Search for more papers by this authorCorresponding Author
Prof. Nagao Kobayashi
Department of Chemistry and Materials, Faculty of Textile Science and Technology, Shinshu University, Ueda, 386-8567 Japan
Search for more papers by this authorCorresponding Author
Prof. Dennis K. P. Ng
Department of Chemistry, The Chinese University of Hong Kong, Shatin, N.T., Hong Kong, China
Search for more papers by this authorGraphical Abstract
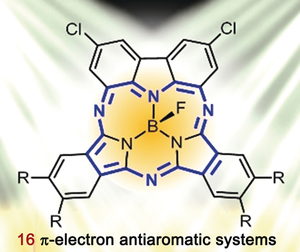
Core competencies: A series of novel core-expanded boron(III) subphthalocyanine analogues, namely boron(III) carbazosubphthalocyanines, have been synthesized and characterized. They represent the first examples of antiaromatic boron(III) subphthalocyanine derivatives and the smallest antiaromatic azaporphyrinoids reported thus far.
Abstract
Condensation of 1,8-diamino-3,6-dichlorocarbazole with a series of disubstituted 1,3-diiminoisoindolines, followed by treatment with BF3⋅OEt2 led to the formation of the corresponding core-expanded boron(III) subphthalocyanine analogues. These air-stable π-conjugated boron(III) carbazosubphthalocyanines possess two boron-containing seven-membered-ring units and a 16 π-electron skeleton, and represent the first examples of antiaromatic boron(III) subphthalocyanine analogues as supported by spectroscopic and theoretical studies. The molecular structure of one of these compounds was unambiguously determined by single-crystal X-ray diffraction analysis. In contrast to typical boron(III) subphthalocyanines, which adopt a cone-shaped structure, the π skeleton of this compound is almost planar.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201811420-sup-0001-misc_information.pdf4.7 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aY. M. Sung, J. Oh, W.-Y. Cha, W. Kim, J. M. Lim, M.-C. Yoon, D. Kim, Chem. Rev. 2017, 117, 2257–2312;
- 1bT. Tanaka, A. Osuka, Chem. Rev. 2017, 117, 2584–2640;
- 1cB. K. Reddy, A. Basavarajappa, M. D. Ambhore, V. G. Anand, Chem. Rev. 2017, 117, 3420–3443.
- 2J.-Y. Shin, T. Yamada, H. Yoshikawa, K. Awaga, H. Shinokubo, Angew. Chem. Int. Ed. 2014, 53, 3096–3101; Angew. Chem. 2014, 126, 3160–3165.
- 3
- 3aT. Nishinaga, T. Ohmae, K. Aita, M. Takase, M. Iyoda, T. Arai, Y. Kunugi, Chem. Commun. 2013, 49, 5354–5356;
- 3bJ. L. Marshall, K. Uchida, C. K. Frederickson, C. Schütt, A. M. Zeidell, K. P. Goetz, T. W. Finn, K. Jarolimek, L. N. Zakharov, C. Risko, R. Herges, O. D. Jurchescu, M. M. Haley, Chem. Sci. 2016, 7, 5547–5558.
- 4M. D. Peeks, T. D. W. Claridge, H. L. Anderson, Nature 2017, 541, 200–203.
- 5M. Umetani, T. Tanaka, T. Kim, D. Kim, A. Osuka, Angew. Chem. Int. Ed. 2016, 55, 8095–8099; Angew. Chem. 2016, 128, 8227–8231.
- 6
- 6aT. Ito, Y. Hayashi, S. Shimizu, J.-Y. Shin, N. Kobayashi, H. Shinokubo, Angew. Chem. Int. Ed. 2012, 51, 8542–8545; Angew. Chem. 2012, 124, 8670–8673;
- 6bX. Li, Y. Meng, P. Yi, M. Stępień, P. J. Chmielewski, Angew. Chem. Int. Ed. 2017, 56, 10810–10814; Angew. Chem. 2017, 129, 10950–10954;
- 6cT. Yonezawa, S. A. Shafie, S. Hiroto, H. Shinokubo, Angew. Chem. Int. Ed. 2017, 56, 11822–11825; Angew. Chem. 2017, 129, 11984–11987;
- 6dT. Yoshida, K. Takahashi, Y. Ide, R. Kishi, J.-y. Fujiyoshi, S. Lee, Y. Hiraoka, D. Kim, M. Nakano, T. Ikeue, H. Yamada, H. Shinokubo, Angew. Chem. Int. Ed. 2018, 57, 2209–2213; Angew. Chem. 2018, 130, 2231–2235.
- 7R. Nozawa, K. Yamamoto, I. Hisaki, J.-Y. Shin, H. Shinokubo, Chem. Commun. 2016, 52, 7106–7109.
- 8S. P. Panchal, S. C. Gadekar, V. G. Anand, Angew. Chem. Int. Ed. 2016, 55, 7797–7800; Angew. Chem. 2016, 128, 7928–7931.
- 9M. Pawlicki, K. Hurej, L. Szterenberg, L. Latos-Grażyński, Angew. Chem. Int. Ed. 2014, 53, 2992–2996; Angew. Chem. 2014, 126, 3036–3040.
- 10B. Szyszko, A. Białońska, L. Szterenberg, L. Latos-Grażyński, Angew. Chem. Int. Ed. 2015, 54, 4932–4936; Angew. Chem. 2015, 127, 5014–5018.
- 11
- 11aR. Nozawa, H. Tanaka, W.-Y. Cha, Y. Hong, I. Hisaki, S. Shimizu, J.-Y. Shin, T. Kowalczyk, S. Irle, D. Kim, H. Shinokubo, Nat. Commun. 2016, 7, 13620;
- 11bM. H. Chua, T. Kim, Z. L. Lim, T. Y. Gopalakrishna, Y. Ni, J. Xu, D. Kim, J. Wu, Chem. Eur. J. 2018, 24, 2232–2241.
- 12
- 12aJ. A. Cissell, T. P. Vaid, A. G. DiPasquale, A. L. Rheingold, Inorg. Chem. 2007, 46, 7713–7715;
- 12bE. W. Y. Wong, C. J. Walsby, D. B. Leznoff, Inorg. Chem. 2010, 49, 3343–3350.
- 13A. Muranaka, S. Ohira, D. Hashizume, H. Koshino, F. Kyotani, M. Hirayama, M. Uchiyama, J. Am. Chem. Soc. 2012, 134, 190–193.
- 14
- 14aT. Satoh, M. Minoura, H. Nakano, K. Furukawa, Y. Matano, Angew. Chem. Int. Ed. 2016, 55, 2235–2238; Angew. Chem. 2016, 128, 2275–2278;
- 14bA. Yamaji, H. Tsurugi, Y. Miyake, K. Mashima, H. Shinokubo, Chem. Eur. J. 2016, 22, 3956–3961.
- 15T. Furuyama, T. Sato, N. Kobayashi, J. Am. Chem. Soc. 2015, 137, 13788–13791.
- 16
- 16aC. G. Claessens, D. González-Rodríguez, T. Torres, Chem. Rev. 2002, 102, 835–854;
- 16bN. Kobayashi in The Porphyrin Handbook, Vol. 15 (Eds.: ), Academic Press, San Diego, CA, 2003, pp. 161–262;
10.1016/B978-0-08-092389-5.50010-9 Google Scholar
- 16cG. E. Morse, T. P. Bender, ACS Appl. Mater. Interfaces 2012, 4, 5055–5068;
- 16dC. G. Claessens, D. González-Rodríguez, M. S. Rodríguez-Morgade, A. Medina, T. Torres, Chem. Rev. 2014, 114, 2192–2277.
- 17
- 17aH. Zhu, S. Shimizu, N. Kobayashi, Angew. Chem. Int. Ed. 2010, 49, 8000–8003; Angew. Chem. 2010, 122, 8172–8175;
- 17bS. Shimizu, S. Nakano, A. Kojima, N. Kobayashi, Angew. Chem. Int. Ed. 2014, 53, 2408–2412; Angew. Chem. 2014, 126, 2440–2444.
- 18
- 18aF. Fernández-Lázaro, T. Torres, B. Hauschel, M. Hanack, Chem. Rev. 1998, 98, 563–575;
- 18bN. Toriumi, A. Muranaka, K. Hirano, K. Yoshida, D. Hashizume, M. Uchiyama, Angew. Chem. Int. Ed. 2014, 53, 7814–7818; Angew. Chem. 2014, 126, 7948–7952.
- 19J. Guilleme, D. González-Rodríguez, T. Torres, Angew. Chem. Int. Ed. 2011, 50, 3506–3509; Angew. Chem. 2011, 123, 3568–3571.
- 20
- 20aS. Hapuarachchige, G. Montaño, C. Ramesh, D. Rodriguez, L. H. Henson, C. C. Williams, S. Kadavakkollu, D. L. Johnson, C. B. Shuster, J. B. Arterburn, J. Am. Chem. Soc. 2011, 133, 6780–6790;
- 20bB. R. Groves, S. M. Crawford, T. Lundrigan, C. F. Matta, S. Sowlati-Hashjin, A. Thompson, Chem. Commun. 2013, 49, 816–818;
- 20cK. Lee, C. M. Donahua, S. R. Daly, Dalton Trans. 2017, 46, 9394–9406.
- 21M. S. Rodríguez-Morgade, C. G. Claessens, A. Medina, D. González-Rodríguez, E. Gutiérrez-Puebla, A. Monge, I. Alkorta, J. Elguero, T. Torres, Chem. Eur. J. 2008, 14, 1342–1350.
- 22Z. Chen, C. S. Wannere, C. Corminboeuf, R. Puchta, P. v. R. Schleyer, Chem. Rev. 2005, 105, 3842–3888.
- 23
- 23aR. Herges, D. Geuenich, J. Phys. Chem. A 2001, 105, 3214–3220;
- 23bD. Geuenich, K. Hess, F. Köhler, R. Herges, Chem. Rev. 2005, 105, 3758–3772.
- 24
- 24aJ. Fleischhauer, U. Howeler, J. Michl, J. Phys. Chem. A 2000, 104, 7762–7775;
- 24bJ. Fleischhauer, U. Howeler, J. Spanget-Larsen, J. Michl, J. Phys. Chem. A 2004, 108, 3225–3234;
- 24cJ. Fleischhauer, G. Raabe, K. A. Klingensmith, U. Howeler, R. K. Chattrjee, K. Hafner, E. Vogel, J. Michl, Int. J. Quantum Chem. 2005, 102, 925–939.
- 25A. Muranaka, S. Ohira, N. Toriumi, M. Hirayama, F. Kyotani, Y. Mori, D. Hashizume, M. Uchiyama, J. Phys. Chem. A 2014, 118, 4415–4424.
- 26
- 26aJ. Michl, J. Am. Chem. Soc. 1978, 100, 6801–6811;
- 26bJ. Michl, J. Am. Chem. Soc. 1978, 100, 6812–6818;
- 26cJ. Mack, M. J. Stillman in The Porphyrin Handbook, Vol. 16 (Eds.: ), Academic Press, San Diego, 2003, pp. 43–116.
- 27
- 27aA. D. Becke, Phys. Rev. A 1988, 38, 3098–3100;
- 27bC. Lee, W. Yang, R. G. Parr, Phys. Rev. B 1988, 37, 785–789.
- 28P. V. Solntsev, K. L. Spurgin, J. R. Sabin, A. A. Heikal, V. N. Nemykin, Inorg. Chem. 2012, 51, 6537–6547.
- 29CCDC 1890660 (3 a) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.