Efficient Docking–Migration Strategy for Selective Radical Difluoromethylation of Alkenes
Dedicated to Professor John R. Falck on the occasion of his 70th birthday
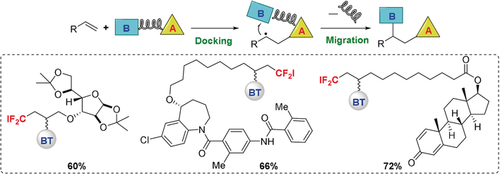
Graphical Abstract
Abstract
Radical-mediated difunctionalization of alkenes is a powerful tactic for olefin utilization. However, the transformation of unactivated alkenes still remains a formidable challenge. Now a conceptually new docking–migration strategy is presented for the difunctionalization of alkenes with photoredox catalysis. Both activated and unactivated alkenes are suitable substrates. A vast array of functional groups are compatible with the mild reaction conditions. Heteroaryl and difluoromethyl are concomitantly incorporated into alkenes, leading to synthetically valuable products that are readily converted into a variety of fluorine-containing molecules. The protocol provides a kinetic control of stereoselectivity for cycloalkenes to generate the unusual cis-products, and offers an efficient approach for the late-stage functionalization of complex natural products and drug molecules. A portfolio of dual-function reagents are prepared for the elusive radical difunctionalization of alkenes.