Pd-Catalyzed Atroposelective C−H Allylation through β-O Elimination: Diverse Synthesis of Axially Chiral Biaryls
Gang Liao
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorBing Li
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorHao-Ming Chen
School of Chemical & Environmental Engineering, Wuyi University, Jiangmen, 529020 China
Search for more papers by this authorQi-Jun Yao
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorYu-Nong Xia
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorJun Luo
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Bing-Feng Shi
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
State Key Laboratory of Elemento-organic Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorGang Liao
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorBing Li
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorHao-Ming Chen
School of Chemical & Environmental Engineering, Wuyi University, Jiangmen, 529020 China
Search for more papers by this authorQi-Jun Yao
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorYu-Nong Xia
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorJun Luo
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Bing-Feng Shi
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
State Key Laboratory of Elemento-organic Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorGraphical Abstract
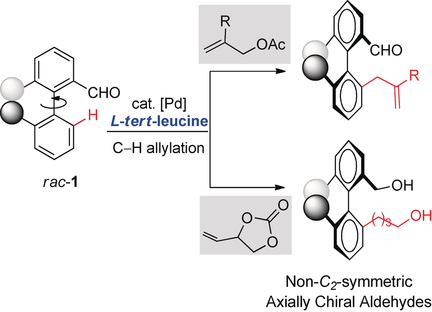
A Pd-catalyzed atroposelective C−H allylation with allylic surrogates is reported. tert-Leucine was identified as an efficient catalytic transient chiral auxiliary. A range of enantioenriched biaryls were prepared in synthetically useful yields with enantioselectivities up to >99 % ee through β-O elimination. The reaction could be scaled up and the products could be further converted into enantiomerically pure axially chiral carboxylic acids.
Abstract
Biaryl atropisomers are of great importance in natural products, pharmaceuticals, and asymmteric synthesis. The efficient synthesis of these chiral scaffolds with full enantiocontrol and high diversity remains challenging. Reported herein is a Pd-catalyzed atroposelective C−H allylation with tert-leucine as an efficient catalytic chiral transient auxiliary. A wide range of enantioenriched biaryl aldehydes were prepared in synthetically useful yields with excellent enantioselectivity (up to >99 % ee) through β-O elimination. The reaction could be carried out on a gram scale without erosion of the ee value. A variety of axially chiral carboxylic acids could be obtained with high enantiopurity. The resulting axially chiral biaryl aldehydes and carboxylic acids might be used in asymmetric synthesis as chiral ligands and/or organocatalysts.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201811256-sup-0001-misc_information.pdf16.6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. Chang, J. Reiner, J. Xie, Chem. Rev. 2005, 105, 4581;
- 1bG. Bringmann, T. Gulder, T. A. M. Gulder, M. Breuning, Chem. Rev. 2011, 111, 563;
- 1cJ. Clayden, W. J. Moran, P. J. Edwards, S. R. LaPlante, Angew. Chem. Int. Ed. 2009, 48, 6398; Angew. Chem. 2009, 121, 6516;
- 1dS. R. LaPlante, P. J. Edwards, L. D. Fader, A. Jakalian, O. Hucke, ChemMedChem 2011, 6, 505.
- 2
- 2a Privileged Chiral Ligands and Catalysts (Ed.: ), Wiley-VCH, Weinheim, 2011;
- 2bR. Noyori, H. Takaya, Acc. Chem. Res. 1990, 23, 345;
- 2cT. Akiyama, J. Itoh, K. Fuchibe, Adv. Synth. Catal. 2006, 348, 999;
- 2dY.-M. Li, F.-Y. Kwong, W.-Y. Yu, A. S. Chan, Coord. Chem. Rev. 2007, 251, 2119.
- 3
- 3aY. Chen, S. Yekta, A. K. Yudin, Chem. Rev. 2003, 103, 3155;
- 3bJ. M. Brunel, Chem. Rev. 2007, 107, PR 1;
- 3cJ. Yu, F. Shi, L.-Z. Gong, Acc. Chem. Res. 2011, 44, 1156;
- 3dD. Parmar, E. Sugiono, S. Raja, M. Rueping, Chem. Rev. 2014, 114, 9047;
- 3eC. Min, D. Seidel, Chem. Soc. Rev. 2017, 46, 5889.
- 4T. Akiyama, J. Itoh, K. Yokota, K. Fuchibe, Angew. Chem. Int. Ed. 2004, 43, 1566; Angew. Chem. 2004, 116, 1592.
- 5D. Uraguchi, M. Terada, J. Am. Chem. Soc. 2004, 126, 5356.
- 6
- 6aT. Hashimoto, K. Maruoka, J. Am. Chem. Soc. 2007, 129, 10054;
- 6bT. Hashimoto, N. Uchiyama, K. Maruoka, J. Am. Chem. Soc. 2008, 130, 14380;
- 6cT. Hashimoto, H. Kimura, K. Maruoka, Angew. Chem. Int. Ed. 2010, 49, 6844; Angew. Chem. 2010, 122, 6996;
- 6dT. Hashimoto, H. Kimura, Y. Kawamata, K. Maruoka, Nat. Chem. 2011, 3, 642;
- 6eY. Ota, Y. Kawato, H. Egami, Y. Hamashima, Synlett 2017, 28, 976.
- 7Q. Wang, Q. Gu, S.-L. You, Angew. Chem. Int. Ed. 2018, https://doi.org/10.1002/anie.201808700; Angew. Chem. 2018, https://doi.org/10.1002/ange.201808700.
- 8
- 8aB. Xu, L.-L. Shi, Y.-Z. Zhang, Z.-J. Wu, L.-N. Fud, C.-Q. Luo, L.-X. Zhang, Y.-G. Peng, Q.-X. Guo, Chem. Sci. 2014, 5, 1988;
- 8bW. Wen, L. Chen, M.-J. Luo, Y. Zhang, Y.-C. Chen, Q. Ouyang, Q.-X. Guo, J. Am. Chem. Soc. 2018, 140, 9774.
- 9J. Chen, X. Gong, J. Li, Y. Li, J. Ma, C. Hou, G. Zhao, W. Yuan, B. Zhao, Science 2018, 360, 1438.
- 10For recent reviews on the synthesis of axially chiral biaryls, see:
- 10aO. Baudoin, Eur. J. Org. Chem. 2005, 4223;
- 10bG. Bringmann, A. J. Price Mortimer, P. A. Keller, M. J. Gresser, J. Garner, M. Breuning, Angew. Chem. Int. Ed. 2005, 44, 5384; Angew. Chem. 2005, 117, 5518;
- 10cT. W. Wallace, Org. Biomol. Chem. 2006, 4, 3197;
- 10dG. Bringmann, D. Menche, Acc. Chem. Res. 2001, 34, 615;
- 10eJ. Wencel-Delord, A. Panossian, F. R. Leroux, F. Colobert, Chem. Soc. Rev. 2015, 44, 3418;
- 10fG. Ma, M. P. Sibi, Chem. Eur. J. 2015, 21, 11644;
- 10gP. Loxq, E. Manoury, R. Poli, E. Deydier, A. Labande, Coord. Chem. Rev. 2016, 308, 131;
- 10hH. Yang, X. Yang, W. Tang, Tetrahedron 2016, 72, 6143;
- 10iB. Zilate, A. Castrogiovanni, C. Sparr, ACS Catal. 2018, 8, 2981;
- 10jA. Link, C. Sparr, Chem. Soc. Rev. 2018, 47, 3804;
- 10kY.-B. Wang, B. Tan, Acc. Chem. Res. 2018, 51, 534.
- 11Reviews on asymmetric C−H functionalization:
- 11aR. Giri, B.-F. Shi, K. M. Engle, N. Maugel, J.-Q. Yu, Chem. Soc. Rev. 2009, 38, 3242;
- 11bL. Yang, H. Huang, Catal. Sci. Technol. 2012, 2, 1099;
- 11cK. M. Engle, J.-Q. Yu, J. Org. Chem. 2013, 78, 8927;
- 11dJ. Wencel-Delord, F. Colobert, Chem. Eur. J. 2013, 19, 14010;
- 11eC. Zheng, S.-L. You, RSC Adv. 2014, 4, 6173;
- 11fJ. Pedroni, N. Cramer, Chem. Commun. 2015, 51, 17647;
- 11gC. G. Newton, S.-G. Wang, C. C. Oliveira, N. Cramer, Chem. Rev. 2017, 117, 8908;
- 11hD.-W. Gao, Q. Gu, C. Zheng, S.-L. You, Acc. Chem. Res. 2017, 50, 351;
- 11iT. G. Saint-Denis, R.-Y. Zhu, G. Chen, Q.-F. Wu, J.-Q. Yu, Science 2018, 359, 759.
- 12F. Kakiuchi, P. L. Gendre, A. Yamada, H. Ohtaki, S. Murai, Tetrahedron: Asymmetry 2000, 11, 2647.
- 13J. L. Gustafson, D. Lim, S. J. Miller, Science 2010, 328, 1251.
- 14
- 14aJ. Zheng, S.-L. You, Angew. Chem. Int. Ed. 2014, 53, 13244; Angew. Chem. 2014, 126, 13460;
- 14bD.-W. Gao, Q. Gu, S.-L. You, ACS Catal. 2014, 4, 2741;
- 14cJ. Zheng, W.-J. Cui, C. Zheng, S.-L. You, J. Am. Chem. Soc. 2016, 138, 5242.
- 15
- 15aT. Wesch, F. R. Leroux, F. Colobert, Adv. Synth. Catal. 2013, 355, 2139;
- 15bC. K. Hazra, Q. Dherbassy, J. Wencel-Delord, F. Colobert, Angew. Chem. Int. Ed. 2014, 53, 13871; Angew. Chem. 2014, 126, 14091;
- 15cQ. Dherbassy, G. Schwertz, M. Chessé, C. K. Hazra, J. Wencel-Delord, F. Colobert, Chem. Eur. J. 2016, 22, 1735;
- 15dQ. Dherbassy, J.-P. Djukic, J. Wencel-Delord, F. Colobert, Angew. Chem. Int. Ed. 2018, 57, 4668; Angew. Chem. 2018, 130, 4758.
- 16
- 16aZ.-J. Jia, C. Merten, R. Gontla, C. G. Daniliuc, A. P. Antonchick, H. Waldmann, Angew. Chem. Int. Ed. 2017, 56, 2429; Angew. Chem. 2017, 129, 2469;
- 16bG. Shan, J. Flegel, H. Li, C. Merten, S. Ziegler, A. P. Antonchick, H. Waldmann, Angew. Chem. Int. Ed. 2018, 57, 14250; Angew. Chem. 2018, 130, 14446.
- 17
- 17aC. G. Newton, E. Braconi, J. Kuziola, M. D. Wodrich, N. Cramer, Angew. Chem. Int. Ed. 2018, 57, 11040; Angew. Chem. 2018, 130, 11206;
- 17bY.-S. Jang, Ł. Woźniak, J. Pedroni, N. Cramer, Angew. Chem. Int. Ed. 2018, 57, 12901; Angew. Chem. 2018, 130, 13083.
- 18F.-L. Zhang, K. Hong, T.-J. Li, H. Park, J.-Q. Yu, Science 2016, 351, 252.
- 19H. Park, P. Verma, K. Hong, J.-Q. Yu, Nat. Chem. 2018, 10, 755.
- 20
- 20aS.-Y. Yan, Y.-Q. Han, Q.-J. Yao, X.-L. Nie, L. Liu, B.-F. Shi, Angew. Chem. Int. Ed. 2018, 57, 9093; Angew. Chem. 2018, 130, 9231;
- 20bG. Liao, Q.-J. Yao, Z.-Z. Zhang, Y.-J. Wu, D.-Y. Huang, B.-F. Shi, Angew. Chem. Int. Ed. 2018, 57, 3661; Angew. Chem. 2018, 130, 3723;
- 20cQ.-J. Yao, S. Zhang, B.-B. Zhan, B.-F. Shi, Angew. Chem. Int. Ed. 2017, 56, 6617; Angew. Chem. 2017, 129, 6717.
- 21
- 21aB. M. Trost, D. L. Van Vranken, Chem. Rev. 1996, 96, 395;
- 21bB. M. Trost, M. L. Crawley, Chem. Rev. 2003, 103, 2921;
- 21cD. Basavaiah, K. Venkateswara Rao, R. Jannapu Reddy, Chem. Soc. Rev. 2007, 36, 1581;
- 21dZ. Lu, S. Ma, Angew. Chem. Int. Ed. 2008, 47, 258; Angew. Chem. 2008, 120, 264;
- 21eD. Basavaiah, B. S. Reddy, S. S. Badsara, Chem. Rev. 2010, 110, 5447;
- 21fJ. F. Hartwig, L. M. Stanley, Acc. Chem. Res. 2010, 43, 1461;
- 21gM. Yus, J. C. Gonzalez-Gomez, F. Foubelo, Chem. Rev. 2011, 111, 7774;
- 21hB. Sundararaju, M. Achard, C. Bruneau, Chem. Soc. Rev. 2012, 41, 4467;
- 21iT.-Y. Liu, M. Xie, Y.-C. Chen, Chem. Soc. Rev. 2012, 41, 4101;
- 21jA. Lumbroso, M. L. Cooke, B. Breit, Angew. Chem. Int. Ed. 2013, 52, 1890; Angew. Chem. 2013, 125, 1942;
- 21kY. Wei, M. Shi, Chem. Rev. 2013, 113, 6659;
- 21lN. A. Butt, W. Zhang, Chem. Soc. Rev. 2015, 44, 7929;
- 21mK. Spielmann, G. Niel, R. M. de Figueiredoa, J.-M. Campagne, Chem. Soc. Rev. 2018, 47, 1159.
- 22N. K. Mishra, S. Sharma, J. Park, S. Han, I. S. Kim, ACS Catal. 2017, 7, 2821.
- 23Suitable crystals were selected for measurement on an Oxford Diffraction Gemini A Ultra diffractometer with CuKα radiation (λ=1.54178 Å). CCDC 1877116 and 1877121 (5 f, 6 j) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.